|
Disease - population management
"In 1950
they bred 100 million mosquitoes a month at the Dominion Parasite
Laboratory in Belleville Ontario. They were contaminated with
bacterial crystalline agents (now believed to be the mycoplasma) at
Queen's University under the direction of Biology head, Dr. Guilford
B. Reed, and then tested by the U. S and Canadian Military in
various Canadian and American communities."
Rose Stevens Director of Vaccine Safety
Concerns for the CCMRF
|
A Short History Of Secret US Human Biological Experimentation.
|
1931: Dr. Cornelius Rhoads, under the auspices of the Rockefeller Institute for Medical Investigations, infects human subjects with cancer cells. He later goes on to establish the U.S. Army Biological Warfare facilities in Maryland, Utah, and Panama, and is named to the U.S. Atomic Energy Commission. While there, he begins a series of radiation exposure experiments on American soldiers and civilian hospital patients.
1932: The Tuskegee Syphilis Study begins. 200 black men diagnosed with syphilis are never told of their illness, are denied treatment, and instead are used as human guinea pigs in order to follow the progression and symptoms of the disease. They all subsequently die from syphilis, their families never told that they could have been treated.
1935: The Pellagra Incident. After millions of individuals die from Pellagra over a span of two decades, the U.S. Public Health Service finally acts to stem the disease. The director of the agency admits it had known for at least 20 years that Pellagra is caused by a niacin deficiency but failed to act since most of the deaths occured within poverty-striken black populations.
1940: Four hundred prisoners in Chicago are infected with Malaria in order to study the effects of new and experimental drugs to combat the disease. Nazi doctors later on trial at Nuremberg cite this American study to defend their own actions during the Holocaust.
1942: Chemical Warfare Services begins mustard gas experiments on approximately 4,000 servicemen. The experiments continue until 1945 and made use of Seventh Day Adventists who chose to become human guinea pigs rather than serve on active duty.
1943: In response to Japan's full-scale germ warfare program, the U.S. begins research on biological weapons at Fort Detrick, MD.
1944: U.S. Navy uses human subjects to test gas masks and clothing. Individuals were locked in a gas chamber and exposed to mustard gas and lewisite.
1945: Project Paperclip is initiated. The U.S. State Department, Army intelligence, and the CIA recruit Nazi scientists and offer them immunity and secret identities in exchange for work on top secret government projects in the United States.
1945: "Program F" is implemented by the U.S. Atomic Energy Commission (AEC). This is the most extensive U.S. study of the health effects of fluoride, which was the key chemical component in atomic bomb production. One of the most toxic chemicals known to man, fluoride, it is found, causes marked adverse effects to the central nervous system but much of the information is squelched in the name of national security because of fear that lawsuits would undermine full-scale production of atomic bombs.
1946: Patients in VA hospitals are used as guinea pigs for medical experiments. In order to allay suspicions, the order is given to change the word "experiments" to "investigations" or "observations" whenever reporting a medical study performed in one of the nation's veteran's hospitals.
1947: Colonel E.E. Kirkpatrick of the U.S. Atomic Energy Comission issues a secret document (Document 07075001, January 8, 1947) stating that the agency will begin administering intravenous doses of radioactive substances to human subjects.
1947: The CIA begins its study of LSD as a potential weapon for use by American intelligence. Human subjects (both civilian and military) are used with and without their knowledge.
1950: Department of Defense begins plans to detonate nuclear weapons in desert areas and monitor downwind residents for medical problems and mortality rates.
1950: In an experiment to determine how susceptible an American city would be to biological attack, the U.S. Navy sprays a cloud of bacteria from ships over San Franciso. Monitoring devices are situated throughout the city in order to test the extent of infection. Many residents become ill with pneumonia-like symptoms.
|
1951: Department of Defense begins open air tests using disease-producing bacteria and viruses. Tests last through 1969 and there is concern that people in the surrounding areas have been exposed.
1953: U.S. military releases clouds of zinc cadmium sulfide gas over Winnipeg, St. Louis, Minneapolis, Fort Wayne, the Monocacy River Valley in Maryland, and Leesburg, Virginia. Their intent is to determine how efficiently they could disperse chemical agents.
1953: Joint Army-Navy-CIA experiments are conducted in which tens of thousands of people in New York and San Francisco are exposed to the airborne germs Serratia marcescens and Bacillus glogigii.
1953: CIA initiates Project MKULTRA. This is an eleven year research program designed to produce and test drugs and biological agents that would be used for mind control and behavior modification. Six of the subprojects involved testing the agents on unwitting human beings.
1955: The CIA, in an experiment to test its ability to infect human populations with biological agents, releases a bacteria withdrawn from the Army's biological warfare arsenal over Tampa Bay, Fl.
1955: Army Chemical Corps continues LSD research, studying its potential use as a chemical incapacitating agent. More than 1,000 Americans participate in the tests, which continue until 1958.
1956: U.S. military releases mosquitoes infected with Yellow Fever over Savannah, Ga and Avon Park, Fl. Following each test, Army agents posing as public health officials test victims for effects.
1958: LSD is tested on 95 volunteers at the Army's Chemical Warfare Laboratories for its effect on intelligence.
1960: The Army Assistant Chief-of-Staff for Intelligence (ACSI) authorizes field testing of LSD in Europe and the Far East. Testing of the european population is code named Project THIRD CHANCE; testing of the Asian population is code named Project DERBY HAT.
1965: Project CIA and Department of Defense begin Project MKSEARCH, a program to develop a capability to manipulate human behavior through the use of mind-altering drugs.
1965: Prisoners at the Holmesburg State Prison in Philadelphia are subjected to dioxin, the highly toxic chemical component of Agent Orange used in Viet Nam. The men are later studied for development of cancer, which indicates that Agent Orange had been a suspected carcinogen all along.
1966: CIA initiates Project MKOFTEN, a program to test the toxicological effects of certain drugs on humans and animals.
1966: U.S. Army dispenses Bacillus subtilis variant niger throughout the New York City subway system. More than a million civilians are exposed when army scientists drop lightbulbs filled with the bacteria onto ventilation grates.
1967: CIA and Department of Defense implement Project MKNAOMI, successor to MKULTRA and designed to maintain, stockpile and test biological and chemical weapons.
1968: CIA experiments with the possibility of poisoning drinking water by injecting chemicals into the water supply of the FDA in Washington, D.C.
1969: Dr. Robert MacMahan of the Department of Defense requests from congress $10 million to develop, within 5 to 10 years, a synthetic biological agent to which no natural immunity exists.
1970: Funding for the synthetic biological agent is obtained under H.R. 15090. The project, under the supervision of the CIA, is carried out by the Special Operations Division at Fort Detrick, the army's top secret biological weapons facility. Speculation is raised that molecular biology techniques are used to produce AIDS-like retroviruses.
1970: United States intensifies its development of "ethnic weapons" (Military Review, Nov., 1970), designed to selectively target and eliminate specific ethnic groups who are susceptible due to genetic differences and variations in DNA.
|
1975: The virus section of Fort Detrick's Center for Biological Warfare Research is renamed the Fredrick Cancer Research Facilities and placed under the supervision of the National Cancer Institute (NCI) . It is here that a special virus cancer program is initiated by the U.S. Navy, purportedly to develop cancer-causing viruses. It is also here that retrovirologists isolate a virus to which no immunity exists. It is later named HTLV (Human T-cell Leukemia Virus).
1977: Senate hearings on Health and Scientific Research confirm that 239 populated areas had been contaminated with biological agents between 1949 and 1969. Some of the areas included San Francisco, Washington, D.C., Key West, Panama City, Minneapolis, and St. Louis.
1978: Experimental Hepatitis B vaccine trials, conducted by the CDC, begin in New York, Los Angeles and San Francisco. Ads for research subjects specifically ask for promiscuous homosexual men.
1981: First cases of AIDS are confirmed in homosexual men in New York, Los Angeles and San Francisco, triggering speculation that AIDS may have been introduced via the Hepatitis B vaccine
1985: According to the journal Science (227:173-177), HTLV and VISNA, a fatal sheep virus, are very similar, indicating a close taxonomic and evolutionary relationship.
1986: According to the Proceedings of the National Academy of Sciences (83:4007-4011), HIV and VISNA are highly similar and share all structural elements, except for a small segment which is nearly identical to HTLV. This leads to speculation that HTLV and VISNA may have been linked to produce a new retrovirus to which no natural immunity exists.
1986: A report to Congress reveals that the U.S. Government's current generation of biological agents includes: modified viruses, naturally occurring toxins, and agents that are altered through genetic engineering to change immunological character and prevent treatment by all existing vaccines.
1987: Department of Defense admits that, despite a treaty banning research and development of biological agents, it continues to operate research facilities at 127 facilities and universities around the nation.
1990: More than 1500 six-month old black and hispanic babies in Los Angeles are given an "experimental" measles vaccine that had never been licensed for use in the United States. CDC later admits that parents were never informed that the vaccine being injected to their children was experimental.
1994: With a technique called "gene tracking," Dr. Garth Nicolson at the MD Anderson Cancer Center in Houston, TX discovers that many returning Desert Storm veterans are infected with an altered strain of Mycoplasma incognitus, a microbe commonly used in the production of biological weapons. Incorporated into its molecular structure is 40 percent of the HIV protein coat, indicating that it had been man-made.
1994: Senator John D. Rockefeller issues a report revealing that for at least 50 years the Department of Defense has used hundreds of thousands of military personnel in human experiments and for intentional exposure to dangerous substances. Materials included mustard and nerve gas, ionizing radiation, psychochemicals, hallucinogens, and drugs used during the Gulf War .
1995: U.S. Government admits that it had offered Japanese war criminals and scientists who had performed human medical experiments salaries and immunity from prosecution in exchange for data on biological warfare research.
1995: Dr. Garth Nicolson, uncovers evidence that the biological agents used during the Gulf War had been manufactured in Houston, TX and Boca Raton, Fl and tested on prisoners in the Texas Department of Corrections.
1996: Department of Defense admits that Desert Storm soldiers were exposed to chemical agents.
1997: Eighty-eight members of Congress sign a letter demanding an investigation into bioweapons use & Gulf War Syndrome.
original
|
| Flashbback:
US Govt Admits Lyme Disease a Bioweapon
Lymerayja
The existence of the Lyme disease epidemic is officially covered up in the UK, its myriad presentations routinely misdiagnosed as everything from "M.E." to MS to hypochondria. This is the first admission by a US government body that the cause is an incapacitating biowar agent.
"SAN ANTONIO (AP) - The $10.6 million Margaret Batts Tobin Laboratory Building will provide a 22,000-square-foot facility to study such diseases as anthrax, tularemia, cholera, lyme disease, desert valley fever and other parasitic and fungal diseases.
The Centers for Disease Control and Prevention identified these diseases as potential bioterrorism agents.".
http://www.msnbc.msn.com/id/10039154/
This is the first admission by a US government body that Lyme disease is a biological warfare agent. This is the reason that hundreds of thousands of men, women and children around the world have been left to rot with wrong diagnoses, or have had their Lyme disease acknowledged but been told that it is an "easily-treated" disease, given 3 weeks' antibiotics, then told to shove off when their symptoms carried on after that.
In Britain the existence of the epidemic is denied completely, and virtually no effort made to warn or educate the public about the dangers of ticks, which carry the bacteria Borrelia burgdorferi.
The Borrelia genus has been a subject of biowar experimentation at least as far back as WW2, when the infamous Japanese Unit 731, which tortured and experimented on live prisoners, studied it.
The reality is, Lyme disease is for many a chronic, horrendous, incapacitating disease producing crippling fatigue, constant pain, loss of memory, possible paralysis, psychosis, blindness and even death.
It was an ideal biowar agent because it evades detection on routine tests, has an enormous range of different presentations, and can mimic everything from ADHD to multiple sclerosis to carpal tunnel syndrome to rheumatoid arthritis to chronic fatigue syndrome (M.E.) to lupus to schizophrenia. Enemy medical staff would never know what had hit them, nor even that ONE illness had hit their population, rather than an unexplained rise in dozens of known conditions.
Honest doctors and scientists who tried to treat or research Lyme disease according to ethical principles have been viciously persecuted by government-backed organisations in the US, Europe and elsewhere. Many specialists in the US were threatened with loss of their license or had anonymous, false allegations sent to the medical board, which tied them up in mountains of paperwork and legal fees...some were forced out of medicine or even driven to suicide.
Instead, medical disinfo agents, most of whom have a background in military/biowarfare units, such as Dr Allen Steere, Mark Klempner, Philip Baker, Edward McSweegan, David Dennis, Alan Barbour etc were enabled to assume top positions in Lyme research , CDC, NIH etc from where they issued false information , covering up the true seriousness and chronic nature of the disease, and comdemned untold numbers to a living hell.
via Lymerayja on indymedia
|
|
on contracting Lymes Disease most people get flu-like symptoms not long after the bite of an infected insect. As little as 2 hours after the bite. And flu-like symptoms, as well as other symptoms
|
Lyme disease is caused by a bacteria that is carried and transmitted through bites from several species of ticks. This disease was first recognized in 1976 and has now occurred in 47 states, including Nebraska. By 1993, Nebraska health officials reported 35 human cases: 11 of these cases were confirmed as having been contracted in Lancaster County.
Early signs of the disease include: a red rash which expands in concentric circles outward from the tick bite producing a "bulls-eye" effect. Later, flu-like symptoms occur which include headache, fever, chills, lethargy, and joint and muscle pain. In advanced untreated cases, there may be arthritis-like symptoms in the knees and shoulders and cardiac abnormalities. In most cases, antibiotics have shown to be an effective treatment of this disease, especially in early stages of the disease. There is no vaccine presently available for humans.
Lyme disease can also infect dogs, horses and cattle. In dogs, Lyme disease can cause fever, joint swelling, pain, arthritis, and lameness. Infected dogs may also exhibit a loss of appetite, depression and lethargy. This disease is rarely fatal in dogs, but it can be debilitating and antibiotic treatments can be long and expensive. A vaccine against Lyme disease for dogs is available from a veterinarian and is an initial series of two shots, followed by a yearly booster shot.
- lancaster.unl.edu
|
The black death plague of the 17th century was transmitted - not by rats but by the fleas / ticks that were living on them
Question: If Avian flu actually exists...is it a Bio-warfare agent which is transmitted via an infected insect which lives on chickens?
|
|
|
Flashback 11/13/2003: Scientists create a virus that reproduces
By Elizabeth Weise USA TODAY
It is the stuff of science fiction and bioethical debates: The creation of artificial life. Up until now, it's largely been just that.
But an important technical bridge towards the creation of such life was crossed Thursday when genomics pioneer Craig Venter announced that his research group created an artificial virus based on a real one in just two weeks' time.
When researchers created a synthetic genome (genetic map) of the virus and implanted it into a cell, the virus became ''biologically active,'' meaning it went to work reproducing itself.
Venter cautioned that the creation of artificial human or animal life is a long way off because the synthetic bacteriophage -- the virus that was created -- is a much simpler life form. Bacteriophages are viruses that infect bacteria.
The project was funded in part by the Department of Energy, which hopes to create microbes that would capture carbon dioxide in the atmosphere, produce hydrogen or clean the environment.
But the questions ethicists have raised about such work are numerous: Should we be playing God? Does the potential for good that new life forms may have outweigh the harm they could do?
Arthur Caplan, who heads the University of Pennsylvania's Center for Bioethics, says yes. This technology ''is impressive. It's powerful and it should be treated with humility and caution,'' Caplan says, ''But we should do it.''
A genome is made up of DNA ''letters,'' or base pairs, that combine to ''spell'' an individual's chromosomes. The human genome project was completed in April.
This summer, researchers at Venter's Institute for Biological Energy Alternatives bought commercially available strands of DNA and, using a new technology, coaxed them together to form a duplicate of the genome of a bacteriophage called phi X.
''It's a very important technical advance,'' says Gerald Rubin, a molecular geneticist at the Howard Hughes Medical Institute. ''You can envision the day when one could sit down at a computer, design a genome and then build it. We're still inventing the tools to make that happen, and this is an important one.''
Venter notes the synthetic bacteriophage has 5,000 base pairs in its genome. The human genome has 3 billion, so similar work in human form probably won't happen in this decade, he says.
To date, the largest genome that was synthesized was the 7,500-base-pair polio virus. But that was only semi-functional and took three years to complete.
The researchers chose to put the new technology into the public domain for all scientists to use. It will appear in the next few weeks on the Web site of the Proceedings of the National Academy of Sciences.
The technology raises safety issues, says David Magnus of Stanford's Center for Biomedical Ethics. Even putting it in the public domain is ''a double-edged sword,'' he says. That presumes that allowing everyone access will keep the good guys ahead of the bad guys. ''It's a gamble. . . . It's a bet that everyone has a stake in,'' he says.
- USA Today
see also: Poor reporting on reproducing virus and the replies and discussion thread
|
|
Deadly 1918 flu reborn for study
By M.A.J. McKENNA - The Atlanta Journal-Constitution - Published on: 10/06/05
Federal and private researchers, including scientists at the Centers for Disease Control and Prevention, have re-created the influenza virus that killed 50 million people in 1918 - in hopes of helping the world prepare for a long-expected next pandemic of flu.
The work, hailed as a stunning scientific achievement, confirms what some scientists have long suspected: The lost 1918 virus was a bird flu that jumped species to attack humans, much like the avian flu strain that has killed at least 60 people in Asia since late 2003. It was conducted in three cities and completed in a high-security Atlanta laboratory.
|
The marker for the grave of Pearl Smalley stands with more than 20 tiny headstones in a corner of the public cemetery in Decatur. All mark the graves of children who died in the 1918 flu pandemic. |
|
Yet again we also see Professor John Oxford on the BBC webpages and on TV...giving dire warnings even though he has publicly stated his concerns at the political use of disease fear-mongering
|
The analysis reveals that the current avian flu strain, known as H5N1, has begun to acquire some of the mutations that apparently made the 1918 virus so lethal - though the researchers cannot say how long it might take for the current strain to accumulate them all.
The resurrection of the 1918 flu, which began with a sprinkle of molecules and ended with a living virus, is not without controversy. It is being questioned both for its inherent risks and for how useful its findings ultimately will be in devising antiviral drugs and vaccines.
The results, released Wednesday in simultaneous publications by the journals Nature and Science, "provide critical clues to the genesis of the 1918 pandemic and why it was so lethal," Drs. Julie Gerberding and Anthony Fauci, the directors of the CDC and the National Institute of Allergy and Infectious Diseases, said in a joint statement. "The findings reveal essential information to help us speed our preparation for - and potentially thwart - the next influenza pandemic."
In the Nature paper, Dr. Jeffery Taubenberger and colleagues from the Armed Forces Institute of Pathology in Washington reveal the complete genome of the 1918 virus, which they retrieved from tissues taken from flu victims: two young soldiers whose autopsy records were stored at their own institution, and a woman whose corpse was disinterred from Alaskan permafrost by a pathologist sympathetic to their efforts.
The group, who were originally outsiders to the tight-knit fraternity of flu virology, rocked the scientific world with a 1997 paper asserting that the long-lost 1918 virus -which vanished decades before the development of techniques that could have described it - could be retrieved.
Wednesday's paper made good on that boast. The group found that the virus was almost completely a bird-flu virus - not, as some had thought, a mixture of segments from both avian and human flus - and possessed a handful of mutations in each of its eight genes that probably occurred as the virus began to infect humans. |
|
And in a second analysis, they compared the 1918 sequence with the genetic sequence of the avian flu now circulating in Asia, finding that the current bird flu shares some of those same mutations.
"In a sense, [the current bird flu] might be going down a similar path to what ultimately led to 1918," Taubenberger said. He suggested that with further research, virologists could provide an early-warning checklist of changes signalling what scientists fear most: bird flu's shift from a hard-to-acquire infection in people to one easily transmitted.
Mutation timeline unclear
It is not possible to say when those changes might emerge, he added. Scientists know the rate at which flu strains collect mutations as they circulate among humans - but they do not know how rapidly flu strains change when they move from one species to another as bird flu has done.
Since late 2003, bird flu has killed or caused the preventive slaughter of more than 100 million domestic fowl and wild birds in Asia. Almost all of the 116 people known to have been sickened by the virus are believed to have been infected by birds. The virus may have passed from person to person in a few cases but not in a sustained way.
The Taubenberger group's genome results were used to create the more controversial piece of research revealed Wednesday: the re-creation of a live virus containing almost all of the genetic components of the 1918 strain.
In a three-cornered collaboration using a process called "reverse genetics," a research group at Mount Sinai School of Medicine in New York used Taubenberger's sequence to re-create individual genes from the 1918 virus, and then passed them to Dr. Terrence Tumpey, a senior microbiologist at the CDC.
Tumpey and his colleagues worked under lab conditions designated "biosafety level 3+," a half-step below those used for the most dangerous organisms known, with additional precautions that are normally applied only to bioterror organisms. They inserted the genes in lab culture cells, where the components self-assembled into a living, reproducing virus.
The group then used the recovered virus in experiments on mice, chicken eggs and cultures of human lung tissue. It killed all the mice within days, as well as chicken embryos normally used to produce quantities of virus for vaccines. And it reproduced rapidly in lung cells, even in cell cultures made to mimic certain body tissues where flu cannot normally grow.
But analysis of the recovered virus did not reveal any single mutation that makes the 1918 flu virus - famous for killing young, healthy victims in days and sometimes in hours - so lethal.
"There is not a smoking gun," said Dr. John Treanor, of the University of Rochester School of Medicine, who studies pandemic vaccine development but was not involved in the research. "What we're seeing is that all the individual genes contribute, but there is no single gene in there that is sufficient to make that virus a killer."
The experiments pinpointed molecular terrain that drugs could be designed to attack, Tumpey said, adding: "I have already gotten messages from other scientists saying, 'I have an idea for targeting this.' "
Scientists hail findings
Scientists with no connection to the flu research hailed its results as a significant achievement in illuminating the historical record, but were divided on its usefulness for future pandemics.
"The studies do not add to ? what we need to do to develop a pandemic vaccine," said Dr. David Fedson, a longtime pharmaceutical researcher who now lives in France. The tasks ahead - formulating a vaccine, getting it licensed and distributing it as widely as possible - are not primarily scientific ones, he said.
Other scientists had mixed views on whether the research should have been undertaken at all. The Federation of American Scientists - which earlier this week criticized the CDC for withholding unrelated flu research data - supported the work, noting the CDC submitted the proposal to several research review committees, as well as the independent National Science Advisory Board for Biosecurity.
The CDC deserves praise for ascertaining in advance that currently available antiviral drugs would have protected researchers from becoming infected and spreading the virus, and also for inviting outside researchers to work at its Atlanta labs rather than distributing the re-created virus, said Dr. Michael Stebbins, the federation's director of biology policy.
But the nonprofit Sunshine Project, which opposes biological weapons research, disagreed. "We see no compelling scientific reason to re-create the virulent virus," said Edward Hammond, the project's director. - ajc.com
|
Possession, Use, and Transfer of Select Agents and Toxins--
Reconstructed Replication Competent Forms of the 1918 Pandemic
Influenza Virus Containing Any Portion of the Coding Regions of All
Eight Gene Segments
AGENCY: Centers for Disease Control and Prevention (CDC), Department of
Health and Human Services (HHS).
ACTION: Interim final rule.
-----------------------------------------------------------------------
SUMMARY: We are adding reconstructed replication competent forms of the
1918 pandemic influenza virus containing any portion of the coding
regions of all eight gene segments to the list of HHS select agents and
toxins. We are taking this action for several reasons. First the
pandemic influenza virus of 1918-19 killed up to 50 million people
worldwide, including an estimated 675,000 deaths in the United States.
Also, the complete coding sequence for the 1918 pandemic influenza A
H1N1 virus was recently identified, which will make it possible for
those with knowledge of reverse genetics to reconstruct this virus. In
addition, the first published study on a reconstructed 1918 pandemic
influenza virus demonstrated the high virulence of this virus in cell
culture, embryonated eggs, and in mice relative to other human
influenza viruses. Therefore, we have determined that the reconstructed
replication competent forms of the 1918 pandemic influenza virus
containing any portion of the coding regions of all eight gene segments
have the potential to pose a severe threat to public health and safety.
DATES: The interim final rule is effective on October 20, 2005. Written
comments must be submitted on or before December 19, 2005.
Centers for Disease Control and Prevention
|
| "Avian flu virus H5N1: No proof for existence, pathogenicity, or pandemic potential; non-'H5N1' causation omitted"
Letter from David Crowe and Torsten Engelbrecht in Medical Hypotheses, (November 2005) (Try your library or medical/university library)
Crowe and Engelbrecht analyse the four papers sent to them by the Friedrick Loeffler Institute (FLI) in response to the following questions.
1. Does H5N1 exist?
2. Is it pathogenic to animals?
3. Is it transmissible and pathogenic to humans, and does it have pandemic potential?
4. Have other causes for observed disease been studied?
The results:
Question 1: An infectious clone can be produced in vitro, but no paper describes purification or full description. One paper author essentially told them further information was classified.
Question 2: Only in extraordinary concentrations was diseased produced.
Question 3: They received an article offering an anecdotal report of a Thai boy who died after treatments with anti-microbials and anti-virals in 1997. They also received a report of Hong-Kong boy who died of Reye's syndrome after treatment with antibiotics and salicylic acid. He had no known contact with poultry. The FLI conceded "'There is no scientific forecasting method that can evaluate the possibility that an influenza virus induces a new pandemic.'" (p.2)
Question 4: No competing theories for disease causation have been considered (such as environmental or pharmaceutical factors.)
They conclude: "Our analysis shows the papers do not satisfy our four basic questions. Claims for H5N1 pathogenicity and pandemic potential need to be challenged further." (p.2)
source
see also Attack of the killer ducks Jim West, David Crowe & Torsten Engelbrecht
|
|
|
Project Bioshield / Bioshield II
Senators Joseph Lieberman (D-Conn.) and Orrin Hatch (R-Utah) introduced the "Project BioShield II Act of 2005" (S. 975) on April 29, 2005. The bill builds upon the first BioShield bill, which was signed into law on July 21, 2004, and authorized $5.6 billion over 10 years to encourage pharmaceutical and biotechnology companies to develop bioterrorism countermeasures. Bioshield II would provide additional liability protections for firms creating vaccines or drugs that could cause injuries. No action has been scheduled on the bill yet.
At a May 11 hearing, Senator Richard Burr (R-N.C.), chairman of the Health, Education, Labor and Pensions Subcommittee on Bioterrorism Public Health Preparedness, said he would take into consideration both S. 975 and S. 3 in crafting his version of BioShield II. S. 3, introduced by Senator Judd Gregg (R-N.H.), amends the Homeland Security Act of 2002 and includes provisions similar to those in S. 975 that encourage companies to develop bioterrorism countermeasures.
|
|
Project Bioshield
The President signed the Project Bioshield Act (P.L. 108-276) into law on July 21, following final approval by the House on July 14. The legislation authorizes funds to encourage pharmaceutical and biotechnology companies to develop bioterrorism countermeasures.
First proposed in the 2003 State of the Union address, Project Bioshield provides $5.6 billion over ten years. The final bill guarantees this funding cannot be diverted for other purposes, but Congress retains discretion over the program's annual appropriations, such as the $890 million approved for FY 2004.
Senators Judd Gregg (R-N.H.), Joe Lieberman (D-Conn.) and Orrin Hatch (R-Utah) are said to be working on "Bioshield II," a bill that will provide liability protections for firms creating vaccines or drugs that could cause injuries.
Provided under the proposed Department of Homeland Security FY 2005 budget is $2.5 billion for Project Bioshield, three times the $890 million provided by Congress in FY 2004. The conference report of the FY 2004 Homeland Security Appropriations bill was signed by the President October 1 (P.L. 108-90) and included $890 million for Project BioShield.
"Project BioShield," first announced in the President's State of the Union address in January, is designed to expand and speed up the availability of vaccines and treatments to combat potential bioterrorism agents. Under the plan, the federal government would provide $6 billion over 10 years to create and produce vaccines and treatments and would guarantee drug companies a buyer for these products. In addition, the Food and Drug Administration would have the authority to expedite the approval process for vaccines and treatments and approve their use, prior to formal approval, in the event of a bioterrorist attack.
- aamc.org
|
Bioshield II would provide additional liability protections
for firms creating vaccines or drugs that could cause injuries.
|
The Dark Side of Project BioShield
Project BioShield, a national security measure proposed by President Bush in 2003 to stockpile drugs and treatments against terrorist threats, was approved by Congress and signed by the president on July 21, 2004. Despite taking 560 days to receive congressional approval, Project BioShield passed the House (421-2) and the Senate (99-0-1) with near unanimous support. The measure will provide $5.6 billion over the next 10 years for the purchase of vaccines and therapeutics against chemical, biological, and radiological attacks. Currently, Project BioShield has $0.9 billion (FY 2004) in available funds that will increase to $2.5 billion in 2005. The procurement process will likely involve the submission of requests for application (RFAs) under the guidance of the Department of Homeland Security. Reportedly, the purchase of next-generation vaccines for smallpox and anthrax is high on the government's list.
But before the government purchases any "anti-terror" products, the items must pass safety and immunogenicity testing in humans, as well as challenge in two animal models. These criteria, although essential for safety and efficacy, raise a number of major issues and concerns for any company interested in participating in Project BioShield. For example, why invest large amounts of resources and capital toward the development of a product that has little commercial value and essentially one potential customer - the U.S. government? The development of a vaccine is an expensive endeavor, costing millions of dollars and years of development. And the market upside is relatively limited. Furthermore, some companies already have a head start on the development of drugs and vaccines via federal funds from the NIH, the DoD, and the Defense Advanced Research Projects Agency (DARPA), for example, potentially giving them an unfair advantage.
Despite the fiscal challenges of developing a new drug, many biopharmaceutical companies are eager to obtain biodefense funding for anti-terror product development for two reasons: patriotism and dual-purpose research. The former is laudable, but patriotism does not produce profits, leading some bottom-line-focused companies to use federal funds for the development of highly related, or dual-purpose, research projects. Some companies may use federal funds to develop both a "patriotic" but less profitable product and, at the same time, a commercially viable product.
Lingering Questions
BioShield funding is unique since it awards money to companies after the product has been developed. Thus, companies must fund their own R&D efforts, including preclinical and clinical tests. Interestingly, the required testing in humans and animals raises another concern: What if the BioShield product doesn't protect the animal model from disease or is found to be immunogenic in humans? Negative data may disappoint the company, but how will the company's investors react? Negative results will affect the stock price or investors' attitudes toward the company. Is it, therefore, worth the risk to develop a "patriotic" drug or vaccine that may adversely affect the stock price? And if so, should companies developing BioShield products be granted indemnity for those specific programs?
Another concern over Project BioShield is raised by Una Ryan, CEO of AVANT Immunotherapeutics, which is developing a next-generation anthrax vaccine. "What if a company develops an improved anthrax vaccine before ours, but it does not possess all of the advantages as AVANT's? What happens to ours?" AVANT is developing a single, oral-dose "sip-and-go" combination anthrax-plague vaccine that is temperature stable and provides immunity in days instead of the current multiple-dose anthrax vaccine that may take months. The advantages of AVANT's vaccine may be clear, but others will find themselves in a similar position. Will more than one company be awarded contracts for similar products, or is the first one to the finish line the only winner?
Several other key questions remain. Since many companies already receive funds from federal sources for various biodefense projects and have close ties with government agencies, how fair will the RFA process be? And what guarantees exist that anti-terror products developed will be purchased?
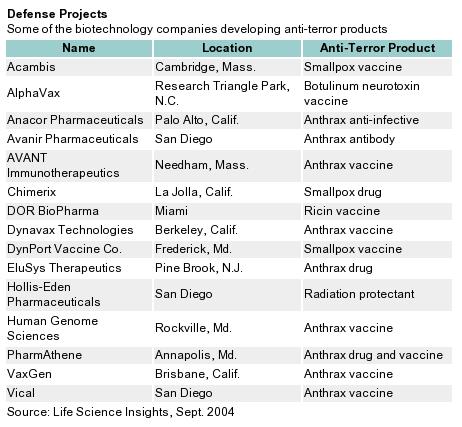
Despite the uncertainties, an estimated 100 biopharma companies are developing anti-terror technologies. The passage of Project BioShield is an important development for the safety of American citizens that provides a significant impetus for drug and vaccine research. That said, Project BioShield might have adverse effects on struggling companies. Given the magnitude of this program and the lingering questions concerning its implementation, it is no surprise that researchers and legislators are refining the program and hope to create a BioShield II in the near future.
Zachary Zimmerman
|
|
who's in charge? yet another lawyer
This story may sound very familiar.
The National Response Plan (NRP), whose formulation was headed by the Department of Homeland Security, is intended to serve as the blueprint to the response to a host of possible disasters and terrorist attacks. The NRP contains several annexes which serve as situation-based response plans called Emergency Support Functions. "Function #8 is the Public Health and Medical Services Annex and it tasks the Secretary of the Department of Health and Human Services (HHS) leadership in responding to a health crisis, such as a flu pandemic, through the Assistant Scretary for Public Health Emergency Preparedness (ASPHEP).
The former Assistant Secretary, Jerome Hauer, was also the director of the Response to Emergencies and Disasters Institute at The George Washington University. Prior to being appointed as assistant secretary, Hauer served as Director of Emergency Management for New York City. Hauer is a gradaute of the Johns Hopkins University School of Public Health and has served on the National Academy of Sciences Institute of Medicine's Committee to Evaluate R&D Needs for Improved Civilian Medical Response to Chemical and Biological Terrorism.
His succesor, appointed in 2003 as ASPHEP, is Stewart Simonson. Like Michael Brown at FEMA, Simonson is a lawyer who was close to a political benefactor. Simonson graduated from the University of Wisconsin law school in 1994 and served as legal counsel to Tommy Thompson while he was governor of Wisconsin from 1995 to 1999. Simonson then followed Thompson to Washington when the governor was appointed as head of HHS. Simonson's bio at HHS states that "from 2001-2003, he was the HHS Deputy General Counsel and provided legal advice and counsel to the Secretary on public health preparedness matters. Prior to joining HHS, Simonson served as corporate secretary and counsel for the National Railroad Passenger Corporation (AMTRAK)."
Congressman Henry Waxman has recently pointed to Simonson as an example where Bush has "repeatedly appointed inexperienced individuals with political connections to important government posts, including positions with key responsibilities for public health and safety."
In addition to being very close to Thompson, Simonson has given generously to the Bush political machine. The website, Political Money Line's contribution database shows that he contributed $3,000 to various Bush-Cheney committees in the 2004 election cycle and gave $250 to the RNC. (Which for a $134,000 a year job is more than chump change.)
The Washington Drug Letter published an article in its December 2004 issue in which Hauer was harshly critical of Simonson:
Speaking as part of a biodefense panel in Washington, D.C. Dec. 15, Jerome Hauer, formerly
the Assistant Secretary for Public Health Emergency Preparedness (ASPHEP) at HHS, said the $877 million contract awarded to VaxGen to produce a new anthrax vaccine was insufficient. He also insinuated poor policymaking has left the country vulnerable to terrorist attacks using weapons of mass destruction.
Hauer faulted the current management at the ASPHEP Office, including acting secretary Stewart
Simonson, for not being better prepared to handle its duties. He called for the creation of a new federal
office to coordinate U.S. biodefense activities.
. . .
"The decisions being made do not appear to have a sound basis," said Hauer, currently senior
vice president of government relations for consulting firm Fleishman-Hillard.
Last spring, Simonson came under fire from several Republican senators as well. Idaho Senator Larry Craig, during a Homeland Security Subcommittee hearing in April questioned the acquisition process for influenza vaccine:
Noting that the flu can be lethal to some populations such as the elderly, Sen. Larry Craig, R-Idaho, said the country was unprepared to deal with a possible flu pandemic.
Simonson . . . stopped short of agreeing with Craig's assessment, but said "it would pose an enormous challenge."
Sen. Ted Stevens, R-Alaska, and Gregg also questioned if the process used by Simonson's office to award vaccine development contracts ensured open competition and delivery to prevent a vaccine shortfall.
"Are we creating the same situation with anthrax?" Gregg asked, referring to the flu vaccine shortfall last winter.
Although Simonson said the different agreements show that they are "seeking not to put all our eggs in one basket," he added that he remains unsure if the contract award process is being done right. "We're learning as we go," he said.
The bottom line is that there is a risk of a flu pandemic that could kill millions of people worldwide if it is able to jump from human to human. Hurricane Katrina amply demonstrated what happens when underqualified yet well-connected lawyers are in charge.
- transparentgrid.com
|
|
Bush proposes using military in bird flu pandemic
WASHINGTON, Oct 4 (Reuters) - President George W. Bush suggested using the military to contain any epidemic of avian influenza on Tuesday, saying Congress needs to consider the possibility. He said the military, perhaps the National Guard, might be needed to enforce quarantines if the feared H5N1 bird flu virus changes enough to cause widespread human infection.
"If we had an outbreak somewhere in the United States, do we not then quarantine that part of the country? And how do you, then, enforce a quarantine?" Bush asked at a news conference. "It's one thing to shut down airplanes. It's another thing to prevent people from coming in to get exposed to the avian flu. And who best to be able to effect a quarantine?" Bush added. "One option is the use of a military that's able to plan and move. So that's why I put it on the table. I think it's an important debate for Congress to have."
Bird flu has killed more than 60 people in four Asian nations since late 2003 and has been found in birds in Russia and Europe.
Experts fear that the H5N1 bird flu virus, which appears to be highly fatal when it infects people, will develop the ability to pass easily from person to person and would cause a pandemic that would kill millions.
He noted that some governors may object to the federal government commandeering the National Guard, which is under state command in most circumstances.
"But Congress needs to take a look at circumstances that may need to vest the capacity of the president to move beyond that debate. And one such catastrophe or one such challenge could be an avian flu outbreak," Bush said.
Health experts are working to develop vaccines that would protect against the H5N1 strain of flu, because current influenza vaccines will not.
And countries are also developing stockpiles of drugs that can reduce the risk of serious disease or even sometimes prevent infection -- but supplies and manufacturing capacity are both limited.
Bush said he was concerned and involved in planning for an influenza pandemic, which experts say will definitely come, although they cannot predict when or whether it wil be H5N1 or some other virus.
"And I think the president ought to have all options on the table to understand what the consequences are -- all assets on the table, not options -- assets on the table to be able to deal with something this significant," he said. - alertnet.org
|
hmmm this seems like another mandate for martial law
|
Hospitals to treat avian flu epidemic same as terrorist attack
Thursday, October 13, 2005 - By Patrick Ferrell, The Star
If the avian flu breaks out in the United States, area hospital officials say they will handle the matter much the same as they would a terrorist attack.
"We've all made some type of provisions for how we would handle it. We're correlating it to a weapon of mass destruction type thing," said Bernie Heilicser, an Ingalls Memorial Hospital doctor and medical director of South Cook County Emergency Medical Services. Heilicser said hospitals have been practicing and preparing for bio-terrorism attacks for many years, so things should run smoothly if the deadly avian flu virus impacts the United States.
Since 2003, a strain of avian - or bird - flu has infected poultry throughout Southeast Asia. So far, human infections from poultry have been limited, with an estimated 110 cases and 65 deaths, most of them in Vietnam, according to international media. But the virus is now moving to Eastern Europe, where this week in Turkey and Romania officials slaughtered thousands of fowl to prevent the spread of the disease. Health officials worry the virus may mutate, making a human-to-human infection possible. Since there is yet no vaccine for the avian virus, a mutation could cause a worldwide flu pandemic, health officials say.
The likelihood of a human flu pandemic similar to one that struck in 1918 is very high, U.S. Health and Human Services Secretary Michael Leavitt said this week as he coordinated plans in Asia to deal with the disease. The 1918 pandemic killed an estimated 40 million to 50 million people worldwide; it killed 500,000 in the United States, according to the national Center for Disease Control and Prevention.
"A lot of people look at not will (a mutation and pandemic) happen, but when it will happen," said Karen Martin, manager of infection control at Advocate Christ Medical Center in Oak Lawn. Martin is a member of the Region 7 Bio-Terrorism Task Force. The avian flu does not have a vaccine; the creation of one could take months, if not years, after the first human-to-human case is identified. So the key to stopping the virus' spread will be to quarantine the infected while they are treated. Area hospitals are beginning to look at where they may have "surge capacity," or an area to house infected patients.
Christ, for instance, is looking at the possibility of using its adjacent Hope Children's Hospital as a flu ward if necessary. Other area hospitals are looking at the possibility of creating mobile hospitals in tents. Christ also is stockpiling respirators, masks and individual transport units that will allow doctors to transport avian flu patients without infecting others.
The national Center for Disease Control and Prevention is expected in coming days to release a report dictating how hospitals should respond to an avian flu crisis. President George Bush last week hinted that should an outbreak of human-to-human avian flu occur in the United States, he would use the military to force a quarantine of the infected area. While some envisioned a police state representative of communist countries, such a quarantine could mean the difference between a minor outbreak or a major pandemic.
"That's been the plan for many, many years for any communicable disease," Heilicser said. "The big question is how our government can effectively quarantine the area." Heilicser said such a quarantine is not likely to be enforced by the military, but people will voluntarily comply. "If there is a problem, people would need to pay attention and cooperate," he said. "If we panic, then bad things are going to happen."
A quarantine also could mean that area schools and businesses close for some time to prevent the public spread of the disease. Limiting travel to and from an infected area will be key to limiting the spread of an outbreak, Martin said. "We have so much travel going on in our society that it is going to be very difficult to control a virus," Martin said. Such traveling restrictions along with voluntary and forced quarantines at hospitals were effective at stopping the deadly SARS outbreak in Toronto in 2003. As far as the avian flu is concerned, common sense will always prevail, Martin and Heilicser said.
They suggest people make sure to get a regular flu shot. While the shot won't prevent the spread of the avian flu, it will prevent the typical flu strain. That could make identifying any symptoms and illnesses easier.
"Preventive medicine really has a big plus here," Heilicser said. Such prevention includes washing your hands frequently, covering your mouth and nose when you sneeze or cough and avoiding close contact with those that may be infected. Also, don't be afraid to take a day off.
"They key in a lot of this is people staying home from work," Martin said. "You don't want to be out in public if you're sick."
Patrick Ferrell
|
|
TamiFlu a Roche patent - is this for real???
|
Board of Directors: John Irving Bell, Rolf Hänggi, Peter Brabeck-Letmathe, Bruno Gehrig, André Hoffmann, Franz B. Humer, Lodewijk J.R. de Vink, DeAnne Julius, Walter Frey, Andreas Oeri, Horst Teltschik (from left).
|
The founder of Roche, Fritz Hoffmann-La Roche, was a pioneering entrepreneur who was convinced that the future belonged to branded pharmaceutical products. He was among the first to recognise that the industrial manufacture of standardised medicines would be a major advance in the fight against disease.
This led him to found F. Hoffmann-La Roche & Co. on October 1st 1896. From the very beginning, Fritz Hoffmann attached great importance to product information as the link between the pharmaceutical manufacturer and doctors, pharmacists and patients. Shortly after the foundation of the company, affiliates were opened in Germany, Italy, France, the US, Great Britain and Russia.
Since then, Roche has grown into one of the world's leading healthcare companies and one of the most important in Europe.
|
|
Further expansion of Tamiflu manufacturing capacity
Roche reiterates willingness to enter discussions with governments and other manufacturers on the production of Tamiflu for emergency pandemic use.
Roche announced today that the Food and Drug Administration (FDA) has granted approval of an additional capsule manufacturing site in the US for the supply of the influenza antiviral Tamiflu (oseltamivir), expanding its already significantly increased worldwide production capacity.
This facility is part of a network of more than a dozen production sites for Tamiflu worldwide, more than half of which are with third party manufacturers.
William M. Burns, CEO Roche Pharma Division, commented: "For Tamiflu, the key need today is the rapid expansion of production capacity. Patients' needs in case of a pandemic remain our top priority. We have already significantly expanded production capacity internally and by working in close collaboration with other companies, and we will continue to do so. In addition, we are prepared to discuss all available options, including granting sub-licenses, with any government or private company who approach us to manufacture Tamiflu or collaborate with us in its manufacturing. In support of the global effort to fight a potential pandemic, we would be prepared to discuss such sub-licenses to increase the manufacturing of Tamiflu, provided such groups can realistically produce substantial amounts of the medicine for emergency pandemic use, in accordance with appropriate quality specifications, safety and regulatory guidelines".
Tamiflu is designed to be active against all clinically relevant influenza viruses and key international research groups have demonstrated, using animal models of influenza that Tamiflu is effective against the avian H5N1 strain circulating in the Far East. As a result, more governments are stockpiling Tamiflu therefore Roche is expanding a collaborative production network to meet the increasing demand. The manufacturing process for Tamiflu is complex and lengthy.
Roche has been working with many governments over the last few months to determine their needs for stockpiling of Tamiflu and has received and/or fulfilled orders from around 40 countries.
About Tamiflu (oseltamivir)
Tamiflu is designed to be active against all clinically relevant influenza viruses.3 It works by blocking the action of the neuraminidase (NAI) enzyme on the surface of the virus. When neuraminidase is inhibited, the virus is not able to spread to and infect other cells in the body.
Tamiflu delivers:
* 38 percent reduction in the severity of symptoms1
* 67 percent reduction in secondary complications such as bronchitis, pneumonia and sinusitis in otherwise healthy individuals2
* 37 percent reduction in the duration of influenza illness5,3
* Tamiflu was shown to provide up to 89 percent overall protective efficacy against clinical influenza in adults and adolescents who had been in close contact with influenza-infected patients4
In children, Tamiflu delivers:
* 36 percent reduction in the severity and duration of influenza symptoms5
* 44 percent reduced incidence of associated otitis media as compared to standard care6
As with any antiviral, a theoretical potential exists for an influenza virus to emerge with decreased sensitivity to a drug. Extensive monitoring, by Roche and the independently established Neuraminidase Inhibitor Susceptibility Network (NISN) measured the incidence of resistance to NAIs. From around 4000 patients treated with Tamiflu resistance was encountered in 0.4 per cent in adults and 4 per cent in children aged one to 12. This resistant virus was found to be less virulent than the wild type virus and did not affect the course of the illness.
The greatest use of Tamiflu today is in Japan. To illustrate this, there were an estimated 16 million influenza infections in Japan over the 2004/2005 influenza season. Roche estimates that around 6 million of those individuals infected with the influenza virus received Tamiflu. Even with this degree of usage, resistance appears very infrequent.
Avian Influenza and Pandemics
Most avian influenza viruses are not infectious to humans, but, should an avian and a human influenza virus co-infect a human or a pig, the virus strains can join, mutate and create a completely new virus, which may be transmissible from animals to humans, and from humans to humans. Such a strain would be entirely new in composition, so vaccines developed and administered to date to protect humans during seasonal epidemics, would be ineffective against this new strain, leaving the population vulnerable to infection. Experts believe the next influenza pandemic could result from such a mutation of virus strains.
World Health Organisation
The WHO has recommended as part of its Pandemic Preparedness Plan that countries establish stockpiles of antiviral treatments such as Tamiflu, which are effective against all strains of the influenza virus. The Pandemic Preparedness Plan, along with details of the 15 countries that have implemented national plans, can be viewed on the Internet.
About Roche
Headquartered in Basel, Switzerland, Roche is one of the world's leading research-focused healthcare groups in the fields of pharmaceuticals and diagnostics. As a supplier of innovative products and services for the early detection, prevention, diagnosis and treatment of disease, the Group contributes on a broad range of fronts to improving people's health and quality of life. Roche is a world leader in diagnostics, the leading supplier of medicines for cancer and transplantation and a market leader in virology. In 2004 sales by the Pharmaceuticals Division totalled 21.7 billion Swiss francs, while the Diagnostics Division posted sales of 7.8 billion Swiss francs. Roche employs roughly 65,000 people in 150 countries and has R&D agreements and strategic alliances with numerous partners, including majority ownership interests in Genentech and Chugai. - roche.com
|
| Indian Co. Eyes Generic Tamiflu for 2006
BOMBAY, India (AP) - A major Indian pharmaceutical company said Friday it plans to bring a generic version of the anti-influenza drug Tamiflu into the market early next year, filling any potential shortages in event of a bird flu epidemic. The drug is already in short supply following fears of a possible epidemic. But the Swiss pharmaceutical company Roche Holding AG, which makes Tamiflu, has refused to license generic versions of the drug despite pressure from several countries and United Nations Secretary General Kofi Annan.
Dr. Yusuf K. Hamied, chairman of Cipla Ltd., said that his company has already developed the generic version, oseltamivir, which would be much cheaper than Tamiflu -- the only available drug that is effective in treatment of people infected with bird flu. "We have been able to synthesize it. Once the lab work is done things don't take too long," Hamied said in a telephone interview. "We are in the process of scaling up and commercialization. That should be completed next month."
Hamied did not disclose how his company would price the generic brand, but said the company will make it available at "a humanitarian price." "I have always said there should be access to medicine at affordable prices," he said.
A strip of 10 Tamiflu tablets cost about $60, a lot of money for people in Asia where millions earn less than a dollar a day. Patients are advised to take a tablet daily for at least a week and the dosage could extend up to six weeks for people living in epidemic infested areas.
The H5N1 strain of avian flu has been sweeping through poultry populations in Asia since 2003, infecting humans and killing at least 65 people, mostly poultry workers. The virus does not pass from person to person easily, but experts fear the virus could mutate. The 1918 influenza pandemic killed more than 40 million people. Subsequent pandemics in 1957 and 1968 had lower death rates, but caused extreme disruption.
World Health Organization spokesman Dick Thompson declined to comment specifically on Cipla's plans, but said WHO supported the line taken by Annan at a visit to the Geneva-based agency two weeks ago. "We will take the measures to make sure poor and rich have access to the medications and the vaccines required," Annan said at the time.
Scientists in Taiwan have recently said they, too, can produce generic Tamiflu, if patent issues are resolved.
Generic manufacturers cannot legally sell the patented drug in the West and in many countries in Asia, including India, which recently tightened its patent laws. But the laws in many of these countries allow governments to invalidate patents during emergencies and permit sale of generics.
Hamied didn't say how and where he plans to sell his product, but insisted he won't "break the law."
"Anyone who wants the drug can purchase it from us," he said. "May be people in America and Europe would want to buy it from us, but they are governed by their own patents."
Roche declined a direct comment on Cipla's announcement. "We fully intend to remain the sole manufacturer of Tamiflu, together with our partners," said Daniel Piller, a Roche spokesman, in Geneva.
The company had previously said it is increasing Tamiflu's production. The company also insists that making the drug is a very complex process and if any other company was given a license to make generic copy, it would take at least two to three years for the firm to ramp up production.
But Hamied said that was not the case with his company, which has copied dozens of Western drugs, including Pfizer's Lipitor and Viagra, as well as AIDS drugs, approved by the World Health Organization, that are used by hundreds of thousands of HIV-infected people worldwide.
"We have learned a lot in the past 30 years of flexible patent laws in India," he said.
Until January this year, India's patent laws allowed Indian pharmaceutical firms to make cheap versions of expensive Western drugs using a technique different than that of the patented product. But under the new law, Roche could possibly go to an Indian court, challenging any attempt by Cipla -- India's third largest drug manufacturer -- to market generic copies of Tamiflu. - money central.msn
|
Vaccine TamiFlu hoarded by Roche Useless?
|
Girl 'showed drug-resistant case of bird flu'
14/10/2005 - 19:44:47 - The bird flu virus that infected a Vietnamese girl was resistant to the main drug that's being stockpiled in case of a pandemic, a sign that it's important to keep a second drug on hand as well, a researcher said tonight.
He said the finding was no reason to panic.
The drug in question, Tamiflu, still attacks "the vast majority of the viruses out there," said Yoshihiro Kawaoka of the University of Tokyo and the University of Wisconsin-Madison in the US.
The drug, produced by Swiss-based Roche Holding AG, is in short supply as nations around the world try to stock up on it in case of a global flu pandemic.
Kawaoka said the case of resistance in the 14-year-old girl is "only one case, and whether that condition was something unique we don't know." He also said it's not surprising to see some resistance to Tamiflu, because that had also happened with human flu.
The girl's Tamiflu-resistant virus was susceptible to another drug, Relenza, Kawaoka said. He and colleagues report the case in the October 20 issue of the US journal Nature, which released the study today. The researchers conclude that it might be useful to stockpile Relenza as well as Tamiflu.
Both drugs are being stockpiled by the US government.
The girl, who had been caring for an older brother with the disease, had been receiving low doses of Tamiflu as a preventive measure when the virus was isolated in late February. She later fell ill and was given higher doses. She recovered and left the hospital in March.
Kawaoka said it's not clear whether the low preventive dose had encouraged the emergence of drug resistance.
Dr William Schaffner of Vanderbilt University in Tennessee called the report important and said it shows the importance of watching for drug resistance.
"It is not unusual to find the occasional resistant virus," he said. "It could be just a biological oddity, or we could see this more frequently.
"This is a blip on the radar screen, and it surely does mean that we have to keep the radar operative," Schaffner said. "We have to keep testing more viruses."
- IOL
|
|
Bird flu's tendency to mistakes makes it dangerous
Oct 15 (Reuters) - Lab tests detected the deadly H5N1 strain of bird flu in samples from Romanian ducks on Saturday, confirming the virus had arrived for the first time in mainland Europe. The World Health Organization believes it is only a matter of time before the virus develops the ability to pass easily from human to human, possibly causing a catastrophic pandemic.
It is the virus's tendency to make mistakes when replicating itself that makes it so dangerous and unpredictable. Here are some facts about H5N1 avian influenza:
-- The H5N1 strain first emerged in Hong Kong in 1997, causing the death or destruction of 1.5 million birds and sickening 18 people, killing six.
-- It re-emerged in 2003 in South Korea, and has now spread to China, Vietnam, Thailand, possibly Laos, Indonesia, Turkey and perhaps Romania. Japan, Malaysia and South Korea are considered free of H5N1 avian flu after having outbreaks. H5N1 has also been seen in wild birds in Mongolia, Kazakhstan and Russia's Siberia.
-- The outbreaks have led to the death or destruction of an estimated 150 million birds.
-- H5N1 has infected 117 people in four countries and killed 60, according to the World Health Organization. Experts say more people may have been infected but were not ill enough to seek medical attention, so it is not known what the fatality rate is.
-- Avian flu exists almost everywhere. There are 15 subtypes of influenza virus known to infect birds, but the so-called highly pathogenic forms tend to be caused by influenza A viruses of subtypes H5 and H7.
-- Influenza type A viruses are named according to two proteins they carry call hemagglutinin (H) and neuraminidase (N). There are 16 possible "H" variations and nine "N."
-- Influenza viruses are RNA viruses, meaning they lack mechanisms for proofreading and repairing genetic errors. This makes them especially prone to mutation. This is why there is a new strain of seasonal flu almost every year and why the annual vaccine must be reformulated every year.
-- Some years this means the flu is not especially deadly but it usually kills 250,000 people at a minimum globally, in an average season. About every 20 years or so the virus changes enough to cause a pandemic that infects and kills many more people than usual.
-- Three pandemics occurred in the 20th century -- the 1918 pandemic that killed anywhere between 20 million and 100 million people globally, the 1957 "Spanish influenza" which killed an estimated 2 million people globally and the 1968 "swine flu" which killed 1 million. Experts agree another pandemic could occur at any time.
-- The seasonal flu vaccine provides no protection against H5N1 avian flu. There is an experimental H5N1 avian flu vaccine but there are only a few thousand doses and it is unlikely to provide perfect protection.
-- H5N1 mutates rapidly and is beginning to show some of the changes that made the 1918 H1N1 flu pandemic so deadly.
-- Four drugs work against influenza. But two older drugs, amantadine and rimantadine, already have minimal activity against H5N1. Two newer drugs work better. Tamiflu, known generically as oseltamivir, was invented by Gilead Sciences and is made and marketed by Swiss drug giant Roche Holdings. Relenza, known generically as zanamivir, was developed by Australia's Biota Holdings and is marketed by GlaxoSmithKline.
Relenza is a powder given via the nose and is considered less desirable than a pill like Tamiflu.
-- Just as bacteria develop resistance to antibiotics, viruses develop resistance to antivirals and H5N1 has become resistant to amantadine. It has also begun to show signs of mutating into a form resistant to Tamiflu.
-- Tamiflu and Relenza, in a class known as neuraminidase inhibitors, do not cure influenza infection but can reduce the severity of illness if given within 48 hours after symptoms begin. They may also help prevent infection if given early.
-- WHO has urged countries to develop preparedness plans, but only around 40 have done so. WHO predicts that most developing countries will have no access to vaccines or antiviral drugs throughout the duration of a pandemic, and experts say developed nations will not have enough to cope well.
- alertnet.org
|
Vaccines are useless...er...does this flu even exist?
|
Vaccines are useless against this virus
By Jeremy Laurance, Health Editor Published: 14 October 2005
There is no vaccine available against bird flu. Existing vaccines are unlikely to be effective against the new strain.
Researchers are working to develop a vaccine targeted at the H5N1 strain but even if it is successful, to manufacture sufficient quantities to protect the world's population from a pandemic will take years. A generic H5N1 vaccine would not prevent infection but it might lessen its severity and save lives. Countries including the UK are relying on the anti-viral drug Tamiflu.
Anti-flu drugs work in a different way from vaccines and can be used against any strain of flu. But they have a limited effect, shortening the course of the illness by a day or two, provided they are taken within 48 hours of infection. In outbreaks of ordinary human flu, they can prevent secondary complications such as pneumonia and reduce infectivity, cutting the rate of spread.
It is hoped that they would be similarly effective against bird flu in humans, saving lives by reducing the severity of the illness. But it is not certain.
The Government has ordered 14.6 million courses of Tamiflu, enough for a quarter of the population, at a cost of £200m, from the manufacturers, the Swiss pharmaceutical company Roche. About 900,000 doses have been delivered.
There is a worldwide shortage of Tamiflu because the raw materials from which it is made are scarce and the manufacturing process is slow and complex. If a human pandemic were to arrive in the UK this winter, the shortage could provoke panic, with hospitals under siege. - independent
|
|
Roche to donate bird flu drug to Romania,Turkey-WSJ
LONDON, Oct 17 (Reuters) - Swiss pharmaceuticals company Roche is donating packs of its anti-influenza drug Tamiflu to Turkey and Romania, the Wall Street Journal newspaper reported on Monday in its online edition.
The news comes as governments in short supply of drugs to combat avian influenza stock up ahead of a possible epidemic after laboratory tests confirmed that the deadly H5N1 strain of the virus has been found in birds from Turkey and Romania.
A Roche spokesman said the pharmaceuticals company is donating 20,000 packs of Tamiflu to Turkey to protect workers who may come into contact with infected poultry, and has given 2,400 packs to Romania, the Journal reported.
Roche, which has said it is increasing production of Tamiflu as quickly as possible, has also given three million packs to the World Health Organization, the paper said.
Tamiflu is the most effective anti-viral drug available for avian flu, and governments are rushing to build up stocks amid fears a virus that has claimed more than 60 lives in Asia since 2003 could mutate into a more deadly form for humans.
Roche said on Wednesday it would outsource some stages of production as it comes under pressure to boost supplies, but added it would not relinquish its patents.
"Roche and its partners fully intend to remain the only manufacturer of Tamiflu and are best qualified to scale up production," spokesman Daniel Piller said on Friday. - alertnet.org
|
|
Report: Tamiflu is 'useless' for avian flu
4th December, 2005 (UPI)
A Vietnamese doctor with experience in treating avian flu says Tamiflu, the drug being stockpiled for treatment of avian flue is useless against the virus.
Dr. Nguyen Tuong Van of the Centre for Tropical Diseases in Hanoi has treated 41 victims of H5N1, following World Health Organization guidelines and administering Tamiflu to her patients. She told the Sunday Times of London the medicine had no effect.
'We place no importance on using this drug on our patients', she said. 'Tamiflu is really only meant for treating ordinary type A flu. It was not designed to combat H5N1.'
The newspaper said the finding casts doubt on the British government's pandemic flu policy. The nation's top medical official, Sir Liam Donaldson, has ordered 15 million doses of Tamiflu be stockpiled.
Van said the only way to keep avian flu patients alive is to support all their vital organs -- including the liver and kidneys -- with modern technology like ventilators and dialysis machines, the Sunday Times reported.
The WHO has acknowledge Tamiflu had not been widely successful in human patients, but said it believes it would have been more effective in many Asian countries if it had been used earlier in the illness.
- Big News Network.com
|
|
Brooks laboratory aids in avian flu detection
Blackanthem.com, BROOKS CITY-BASE, Texas, November 11, 2005
A Brooks City-Base epidemiology laboratory is working to develop more effective and timely methods for detecting avian flu to support a worldwide Air Force surveillance program designed to safeguard American military personnel from a potential outbreak.
The Air Force Institute for Operational Health's Epidemiology Division is at the forefront of an Air Force initiative to create more reliable and faster testing procedures for H5N1, the influenza virus that scientists believe has spread from birds to humans across three continents.
"We're developing assays (DNA testing) to rapidly screen for avian flu," said Maj. David Eddington, a molecular biologist who is the AFIOH Epidemiology Division's microbiology chief. He said Air Force scientists began the process this year of developing new technology to detect avian flu. The new assay uses polymerase chain reaction, or PCR, technology. Additionally, the organization is capable of performing molecular sequencing of the viral genome which helps detect mutations.
Since first appearing in Asian poultry in 1997, the Highly Pathogenic Avian Influenza, as it is scientifically known, mostly has killed people who have been in direct contact with domesticated fowl. The deadly respiratory strain has become zoonotic (jumping from non-human host to people), leading to the first reported human death in 1997 in Hong Kong. Scientists fear that its transmission between humans could trigger a pandemic.
To do that, it would have to mutate. During the last century, mutations in influenza viruses caused pandemics that killed millions of people in 1918, 1957 and 1968. For avian flu to become pandemic, it would have to mix its genes with those from the naturally circulating flu 'A' strain to transform so that it becomes easily transmissible from human to human, said Linda Canas, chief, AFIOH Virology section.
Influenza viruses are known to transform with some frequency. This is why surveillance exists to determine which strain is best for the current vaccine, said Major Eddington.
"With the influenza virus, two different mutations called 'shift' and 'drift' exist," said Major Eddington. He said the drift mutation process involves a small nucleic acid variation that occurs after the virus invades a cell and during replication of its nucleic acid genome. Shift mutations, however, involve larger genetic segments and can occur in naturally circulating flu 'A' strains.
"The problem (leading to potential pandemics) is someone infected with a normal circulating flu 'A' strain is (also) co-infected with avian flu," Major Eddington said. The co-mingling of virus strains creates conditions for gene sharing. This occurs after an infected cell produces gene segments from both strains and they mix together during the process called self assembly. During this process, the virus mutates into a new variation in which humans have no immunity.
"The Air Force has stepped up worldwide avian flu surveillance that includes research sites in South America and Thailand," Ms. Canas said. Since 1997, the Air Force has been executive agent for the laboratory-based Global Emerging Infections System.
This tri-service system relies on a network of global sentinel (early warning) sites, Ms. Canas noted. "Our surveillance data is shared with the Food & Drug Administration's Vaccine and Related Biological Products Advisory Committee," she said.
This information is compared with other surveillance information and used to develop North American flu vaccines. Typically, flu vaccines are composed of two 'A' strains and one 'B' strain. All flu vaccines and anti-flu prescription drugs, such as the FDA-approved prophylactic Tamiflu, are made overseas.
Air Force scientists know that current flu vaccines offer no protection against avian flu. Ms. Canas said, "We have very little information on the use of Tamiflu. The only thing that it seems to help is morbidity (onset of illness). We don't know how it will affect mortality."
By Rudy Purificato
311th Human Systems Wing
|
|
Roche settles dispute with Tamiflu inventor
By Tom Armitage ZURICH, Nov 16 (Reuters) - The main producer of Tamiflu, the drug thought to be the best defence against a possible flu pandemic caused by bird flu, has settled a dispute with the drug's inventor over production and royalties, the companies said on Wednesday.
Governments have been seeking to stockpile the drug as a precaution against the possible outbreak of a human variant of avian flu but drug maker Roche Holding AG has come under pressure over concerns production could fall short.
Under Wednesday's deal the drug's inventor Gilead Sciences Inc will get a greater say in plans to increase production of the drug by farming out parts of the process to third-party producers such as generic drug makers.
Gilead's share of the royalties from Tamiflu sales, which are expected to reach over $1 billion due to government orders, will remain unchanged, although it will no longer have to shoulder the burden of certain manufacturing costs.
"The money is not a great deal," said Claude Zehnder, market analyst at Swiss bank ZKB said. "But it is certainly a good thing that they have agreed this amicably."
The settlement also involves Roche paying the U.S. firm around $62.5 million in reimbursements for so-called cost of goods adjustments backdated to the start of 2004.
Investors in the Swiss drugmaker had feared that the dispute, which broke out in June, could weigh on the firm's shares or scupper Tamiflu sales. Roche's participation certificates were 0.4 percent higher at 194.40 Swiss francs by 0806 GMT, in a flat Swiss market. Gilead threatened in June to end a deal signed in 1996 under which Roche got an exclusive licence to manufacture and sell the antiviral drug worldwide. The U.S. firm claimed Roche had failed to market the drug properly, particularly in the United States, and had not launched the drug as a treatment for seasonal flu in other countries. If Gilead had won, all rights to the drug would have reverted back to the U.S. firm.
Roche has come under pressure from governments around the world to increase its production capacity for the treatment and said last month that it would consider allowing other parties, such as generic drugmakers, to produce the remedy. Gilead will also have a greater say in selling Tamiflu as a seasonal flu treatment and will have the option to co-promote the drug in specialised areas in the United States. However, it will not co-promote the drug in 2006, it said. Roche said that Gilead's royalties would remain unchanged, with the U.S. firm receiving a cut of between 14 and 22 percent based on Roche's annual net sales of the drug. This would work out at 18 to 19 percent for 2005, the firms said. Roche has already sold 859 million Swiss francs ($649.8 million) worth of Tamiflu in the first nine months of 2005.
Roche will pay Gilead the $62.5 million in royalty reimbursements and has said that the U.S. firm could keep $18.2 million which Roche had paid in protest over a dispute on royalty calculations for sales in 2001 to 2003. - alertnet.org
|
|
Chugai says two deaths have possible Tamiflu link
(Reuters) Updated: 2005-11-14 - Japan's Chugai Pharmaceutical Co said on Monday it has reported to the government that two teenage boys exhibited abnormal behaviour that led to their deaths after taking the anti-flu drug Tamiflu, made by Chugai's Swiss parent Roche Holding AG.
The comments come in response to weekend news reports that Japan's health ministry is investigating the deaths of two teenage boys who died in accidents linked to odd behaviour shortly after taking the drug. Health ministry officials were not available for comment.
Shares in Chugai were down 3.1 percent at 2,630 yen on Monday afternoon, compared with a 1.3 percent fall in the Tokyo Stock Exchange's pharmaceutical sector subindex.
Tamiflu, considered one of the best defences against bird flu in humans, might help slow the spread of a much-feared pandemic should the H5N1 flu virus become able to spread from person to person. The Mainichi newspaper and Kyodo News agency reported on Saturday that a 17-year-old high school student jumped in front of a truck in February last year shortly after taking the medicine, while a junior high school student is believed to have fallen from the ninth floor of his apartment building this February.
"We reported these cases to the health ministry as a link between the deaths and the drug could not be ruled out," a Chugai spokesman said. The reports were made separately after each incident, he said.
He said Chugai has included in the literature accompanying the drug a list of side effects such as impaired consciousness, abnormal behaviour and hallucinations and has called doctors' attention to the possible side effects. Kyodo said the ministry is considering issuing a fresh warning about the side effects, following its decision to increase stockpiles of the drug amid growing fears about a possible pandemic.
The Pharmaceuticals and Medical Devices Agency said there were 64 cases of psychological disorders linked to the drug between fiscal 2000 and 2004, according to Kyodo.
Chugai launched Tamiflu in Japan in 2001. During the last flu season it shipped the drug to more than 10 million people.
The Japanese government is planning to boost its target stockpile of Tamiflu to 250 million capsules, up 70 percent from its previous target, to cover treatment for 25 million people. - chinadaily.com
|
| Health officials probe Tamiflu deaths
Thursday 17th November, 2005 (UPI)
Health experts in the United States and Japan are investigating the deaths of 12 Japanese children who took Roche's anti-viral Tamiflu.
Food and Drug Administration officials have not yet commented publicly on a possible link between Tamiflu and the fatalities, but the FDA said four of the fatalities were due to sudden death, which the agency said is unusual in otherwise healthy young people.
Tamiflu is viewed as the best medicine currently available to combat a potential bird-flu pandemic. Governments and corporations worldwide are currently stockpiling the drug as pandemic fears mount.
Tamiflu's Swiss sponsor Roche says the medication has been used to treat more than 33 million patients in 80 countries worldwide. The anti-viral was launched in the United States, Canada and Switzerland between 1999 and 2000.
Tamiflu's developer, Gilead Sciences, said earlier this week it had resolved its dispute with Roche over the marketing of the drug. Under a revised deal, Gilead has the option to co-promote the flu treatment starting next year. - Big News Network.com
|
| Tamiflu Neuropsychiatric Events, Death, Serious Skin Reactions To Be Focus Of Pediatric Committee Meeting
Neuropsychiatric events and deaths with Roche's Tamiflu, as well as serious skin reactions, will be discussed by FDA's Pediatric Advisory Committee meeting for Nov. 18.
FDA has reviewed 75 case reports of adverse events from patients receiving Tamiflu (oseltamivir) for influenza treatment. The reports included eight deaths, 32 neuropsychiatric events and 12 skin/hypersensitivity events. The remaining events were categorized as gastrointestinal, musculoskeletal, abnormal test values, vascular, infectious, and hypothermia. There was also and one cardiac adverse event and an overdose.
The 32 neuropsychiatric adverse events included delirium, hallucinations, convulsions, encephalitis and abnormal behavior, which FDA termed the "most alarming" events in its briefing materials for the meeting.
The abnormal behavior involved two adolescents who "jumped out of the second floor window of their homes after receiving two doses" of Tamiflu, and a third eight-year old boy who ran into the street three hours after receiving his first dose of Tamiflu, FDA said.
The vast majority of the 75 case reports, 92%, originated from Japan where Tamiflu usage is more common. According to IMS Health, approximately 24.4 mil. prescriptions for oseltamivir in Japan were dispensed between 2001 and 2005 while approximately 5.5 mil. prescriptions were filled in the U.S. in the same period. Pediatric prescriptions accounted for 11.6 mil. in Japan vs. 872, 386 in the U.S.
FDA said that there is "insufficient evidence to establish that deaths and neuropsychiatric AEs represent a safety signal associated with Tamiflu." The agency suggested that the "pattern of neuropsychiatric AEs [is] more suggestive of increased AE reporting from Japan, increased use of the drug in Japan, and previously described manifestations of influenza."
There is an increased awareness of influenza in Japan facilitated by public health authorities and an active surveillance of flu-associated encephalitis and encephalopathy that began in the late 1990s.
Roche pointed other differences between Japan and the U.S. In Japan, oseltamivir is often dispensed as a powder in Japan, and mixed at the pharmacy, possibly contributing to higher dosing. The length of dosing may also be different, with Japanese patients possibly stopping administration before the recommended period, contributing to recrudescence (symptom reoccurrence), Roche's briefing documents for the meeting state.
Roche commissioned Ingenix to conduct an analysis from U.S. healthcare insurance claims databases. The study investigated the safety of Tamiflu in 63,000 children (1-12 years old) and concluded that there "was no indication of an increased risk of neuropsychiatric events for children aged 1-12 over that already posed by influenza illness alone," Roche said.
Roche also maintains "that there is no association between Tamiflu use and the deaths or neuropsychiatric events reported. Roche sees no scientific or medical basis for any changes in how Tamiflu is used."
While FDA said that updated labeling for neuropsychiatric events and death is not warranted for Tamiflu, the agency plans to closely monitor the adverse events with the antiviral and report back to the Pediatric Advisory Committee in two years.
The agency is, however, recommending the addition of information to labeling in serious skin reactions with Tamiflu.
"Hypersensitivity and serious skin reactions were identified as a potential safety signal that may need to be strengthened in the current oseltamivir product labeling," FDA said.
"As compared to the neuropsychiatric AE reports that may be related, in part, to an increased recognition of influenza-associated neurologic events in Japan, the cases of skin/hypersensitivity reactions do not appear to be related to a manifestation of influenza illness or increased in a specific population," the agency explained.
FDA will ask the committee to comment on its plans to further monitor Tamiflu adverse events and its proposed relabeling for serious skin reactions.
Separately, the European Medicines Agency's Committee for Medicinal Products for Human Use requested at its Nov. 14-17 meeting that Roche provide a "cumulative safety review of all available data on serious psychiatric disorders, including all case reports with a fatal outcome where Tamiflu was involved," according to a Nov. 17 press release.
The request resulted from two cases of alleged suicides in adolescents reported to the EMEA. The agency stresses that the events could have been from concomitant therapies or the result of high fever.
- fdaadvisorycommittee.com
- Roches briefing document pdf
|
| EU Health Regulator Asks Roche For Tamiflu Safety Data
ZURICH (MarketWatch) -- The European health regulator late Thursday said it asked Roche Holding AG (RHHBY) for safety data on its flu drug Tamiflu.
The Committee for Medicinal Products for Human Use decided at its most recent meeting to ask Roche to provide a cumulative safety review of all available data on serious psychiatric disorders, including all case reports with a fatal outcome where Tamiflu was involved.
The Committee for Medicinal Products for Human Use is the scientific committee of the European Medicines Agency, or EMEA. The EMEA is the European equivalent of the U.S. Food and Drug Administration.
The agency's request follows a posting by the FDA Thursday on its Web site ahead of a panel meeting to discuss the safety of Tamiflu and other drugs used to treat children.
Two cases of alleged suicide, associated with the treatment of influenza, were reported to the EMEA. In both cases the adolescents exhibited abnormal or disturbed behavior, which led to their deaths. So far, no causal relationship has been identified between the use of Tamiflu and psychiatric symptoms,such as hallucination and abnormal behaviour, the agency said.
"The EMEA stresses that the assessment of psychiatric events during Tamiflu treatment is difficult, because other medicines are often taken at the same time as Tamiflu and because patients with influenza and a high fever can show psychiatric symptoms."
The agency also said this is particularly relevant for children and elderly patients.
Roche moved to defend its Tamiflu anti-viral drug Thursday, saying only one out of a million children who had taken it had died, and the drug reduced mortality rates among flu sufferers.
Worldwide, Roche said 32 million people have taken Tamiflu, 89 of whom have died and 130 have had so-called neuropsychiatric events. No children have died in the U.S., said Dr. David Reddy, who heads the company's influenza task force.
Most of the deaths and severe side-effects were observed in Japan, where use of the drug is much more widespread than in Europe or the U.S.
Reddy said in past clinical studies, fewer flu patients who had taken Tamiflu died than those in a comparison group who didn't take the drug. He added "We mustn't forget that influenza itself can be a fatal illness."
- marketwatch
Company Web site: http://www.roche.com
|
|
FluWrap: Tamiflu gets safety nod
By KATE WALKER UPI Correspondent WASHINGTON, Nov. 18 (UPI) -- An FDA advisory panel Friday said that Tamiflu is safe and apparently unrelated to the deaths of 12 Japanese children who took the drug.
The Food and Drug Administration panel did suggest adding warnings about possible serious skin conditions, and said the FDA should review the drug safety profile again in a year. But by a unanimous vote, they said there was no evidence to link the drug to the deaths or to serious psychiatric events in children.
The 12 deaths in the past 13 months included one suicide, four cases of sudden death and four heart attacks. Other deaths involved asphyxiation, pneumonia and acute pancreatitis.
There have also been 32 cases of psychiatric abnormalities, including delusions, hallucinations and delirium, reported in children who had taken Tamiflu. Thirty-one of the cases involving psychiatric episodes occurred in Japan.
Two of the psychiatric cases involved teenagers who jumped from second-floor windows after taking two doses of the drug.
"In many of these cases, a relationship to Tamiflu was difficult to assess because of the use of other medications, presence of other medical conditions, and/or lack of adequate detail. The level of detail in these reports was highly variable and determining the contribution of Tamiflu to the deaths was difficult," an FDA summary said. - upi.com
|
1/2 of all Tamiflu users die in Vietnam
| Deaths cast doubts over Tamiflu
By Andrew Jack in London Published: December 21 2005 18:45
Fresh doubts were cast on the efficacy of Tamiflu as a treatment for bird flu on Wednesday night when one of the world’s most prestigious medical journals published new reports of resistance to the drug and deaths in patients in Vietnam.
Menno de Jong and colleagues from the hospital for tropical diseases in Ho Chi Minh City recorded in the New England Journal of Medicine that four out of eight patients suffering from the H5N1 flu strain and treated with Tamiflu had died, including two who developed resistance.
The reports increase suggested levels of resistance to nearly 10 per cent, or three out of the 31 known human cases of H5N1 treated with Tamiflu, which is marketed by Roche of Switzerland.
The study raises new questions about the drug, which more than 50 governments have ordered in significant quantities in recent months to stockpile as a potential prophylactic and treatment in the case of a flu pandemic.
An accompanying article in the Journal reinforced calls for alternative approaches to treatment for a pandemic, including the stockpiling of the rival drug zanamivir, or Relenza.
Dr Anne Moscona wrote that individuals’ stockpiling of Tamiflu was “potentially dangerous” because it could lead to insufficient doses and inadequate courses of therapy, in turn accelerating the development of resistance.
Roche said it took the reports seriously, and was stepping up its own clinical research on Tamiflu’s use in humans and animals, including work on dosages of twice the current levels for longer periods. It said findings should be ready early next year.
The UK health department said on Wednesday night: “Tamiflu was chosen on the basis of independent expert advice that reflected its efficacy and ease of administration. Internationally, this is agreed as the product of choice. Our antiviral strategy is kept under constant review and we are looking carefully at Relenza as a possible back-up to Tamiflu.”
However, David Reddy, who is responsible for Tamiflu at the company, stressed that any findings on resistance or deaths should be set against the fact that an eventual pandemic strain of H5N1 would be different from the current bird flu virus which has so far infected 139 people and killed 71.
Separately, Roche said on Thursday that the US Food and Drug Administration had approved Tamiflu for preventing influenza in children - a vulnerable group during an outbreak. The company said that when the drug was administered within 48 hours of exposure, the incidence of flu fell from 17 per cent in the group not receiving the drug, to 3 per cent in the group that did.
Roche shares fell 0.8 per cent to Sfr197.2 in early trade. - ft.com
|
60% of the entire US population immunosuppressed?
|
The nasal spray however, because it is a live or weakened virus, should be avoided by certain individuals. Nasal spray recipients should avoid close contact with immunocompromised individuals for at least 21 days. This warning is specifically directed toward those living in the same household with anyone who has a weak or weakened immune system.
As many as 60 percent of the entire US population could be considered immunosuppressed and therefore should not be in close contact with anyone who has received the nasal spray flu vaccine.
Some examples of these individuals are those with eczema, cancer, HIV /AIDS, organ recipients, and those on drugs that cause immunosuppression (such as corticosteroids). Also, the nasal spray is also not recommended for use in patients with a history of reactive-airway problems such as asthma. - podiatry online
|
lots of cash into Roche AG and other pockets
[Gilead the inventor of Tamiflu - has Rumsfeld has Rumsfeld as a stockholder]
|
|
Flashback: 2005 avian flu scares
US Defense Secretary Donald Rumsfeld, former chairman of Gilead, the manufacturer of Tamiflu, will also make big profits, since he is a major shareholder.
Better yet, Bilderberger spokesman Etienne F. Davignon (Vice-Chairman, Suez-Tractebel)and Reagan-Bush Cabal insider former Secretary of State George P. Shultz, PhD (Distinguished Fellow, Hoover Institution, Stanford University) are also on the board of directors of Gilead. (http://www.gilead.com/wt/sec/bod)
Another Bilderberger regular is Lodewijk J.R. de Vink, who sits on the board of Hoffman-La Roche, Gilead's partner.
http://www.roche.com/home/company/com_gov/com_gov_dir/com_gov_dir_vink.htm
In other words, the "Bird Flu" scam will generate outrageous profits for globalist-insiders like Shultz, Rumsfeld, Davignon, and de Vink.
- globalnewsmatrix
|
|
|
Rumsfeld's legacy
From mass vaccination to aspartame and back again
Then and current Defense Secretary Donald Rumsfeld was reportedly the architect of the plan to ensure that, "every man, woman and child" in America be vaccinated against the swine flu in 1976.
Within a few weeks, some 47 million Americans were vaccinated with the experimental vaccine. The program resulted in at least 113 deaths, nearly 4,000 cases of paralysis and an epidemic of Guillean Barre -- a polio-like disease. It is impossible to calculate the longterm damage the swine flu disaster has caused in terms of human suffering, chronic disease care and genetic damage being passed on to children.
Insider reports indicate Rumsfeld orchestrated the swine flu fiasco to "add some spark to the campaign of President Ford..." wrote author and marine biologist William Sargent.
In 1978, Rumsfeld became CEO of the G.W. Searle Corp. By June, 1981, his political muscle overcame science so that the deadly neurotoxic artificial sweetener aspartame could achieve U.S. Food and Drug Administration (FDA) approval.
The FDA has linked aspartame to 92 symptoms including paralysis, blindness, neuroses, sexual dysfunction, asthma, diabetes, chronic fatigue and death. According to the Centers for Disease Control, nearly 500,000 Americans each year are simply dropping dead from cardiac arrest. A growing coalition of physicians and researchers believe aspartame is behind these and many other unexplained deaths.
Rumsfeld was also instrumental in promoting the recent mass smallpox vaccination plan that was ultimately scuttled because too many people were experiencing adverse reactions (including death) to the vaccine.
Rumsfeld is again the secretary of defense and again he promoted a plan to vaccinate every man, woman and child against a manufactured disease scare with an untested experimental vaccine.
Is it just a coincidence this man is involved when programs to poison the entire nation are being planned, promoted and executed? What is the real Rumsfeld "public health" agenda?
- proliberty.com
|
a force multiplier
observe the stockpiles of tamiflu being prepared
some people are bulk buying it
|
Donald Rumsfeld makes $5m from bird flu drug
Daily Times Monitor - Wednesday, April 05, 2006 LAHORE:
US Secretary of Defence Donald Rumsfeld has made more than $5 million from selling shares in the biotechnology firm that discovered and developed Tamiflu, the drug being bought in massive amounts by governments to treat a possible human pandemic of bird flu, reports British newspaper the Independent.
More than 60 countries have so far ordered large stocks of the antiviral medication - the only oral medicine believed to be effective against the deadly H5N1 strain of the bird flu virus.
Tamiflu does not cure the disease, but if taken soon after symptoms appear it can reduce its severity. The drug was developed by a Californian biotech company, Gilead Sciences. It is now made and sold by the giant chemical company Roche, which pays it a royalty on every tablet sold, currently about a fifth of its price.
Rumsfeld was on the board of Gilead from 1988 to 2001, and was its chairman from 1997. He then left to join the Bush administration, but retained a huge shareholding.
The firm made a loss in 2003, the year before concern about bird flu started. Then revenues from Tamiflu almost quadrupled, to $44.6m, helping put the company well into the black. Sales almost quadrupled again, to $161.6m last year. During this time the share price trebled.
Rumsfeld sold some of his Gilead shares in 2004 reaping - according to the financial disclosure report he is required to make each year - capital gains of more than $5m. The report showed that he still had up to $25m-worth of shares at the end of 2004, and at least one analyst believes his stake has grown well beyond that figure, as the share price has soared. Further details are not likely to become known, however, until Rumsfeld makes his next disclosure in May.
The 2005 report showed that, in all, he owned shares worth up to $95.9m, from which he got an income of up to $13m, owned land worth up to $17m, and made $1m from renting it out.
He also had illiquid investments worth up to $8.1m, including in partnerships investing in biotechnology, issuing reproductions of paintings, and operating art galleries in New Mexico and Wyoming. He also has life insurance with a surrender value of up to $5m, and received up to $1m from the DHR Foundation, in which he has assets worth up to $25m, and $773,743 from the Donald H Rumsfeld Trust, in which he has assets of up to $50m.
In a statement to The Independent on Sunday the Pentagon said: "Secretary Rumsfeld has no relationship with Gilead Sciences, Inc beyond his investments in the company. When he became Secretary of Defence in January 2001, divestiture of his investment in Gilead was not required by the Senate Armed Services Committee, the Office of Government Ethics or the Department of Defence Standards of Conduct Office.
"Upon taking office, he recused himself from participating in any particular matter when the matter would directly and predictably affect his financial interest in Gilead Sciences." - Pakistan daily times.com |
|
Cel-Sci stock jumps following Avian flu filing
Baltimore Business Journal - 5th April 2006 -
Stock in Cel-Sci Corp., a Virginia biotech firm with an office in Baltimore, has jumped about $1 per share since April 3, a day before the company filed a patent application for an Avian flu vaccine. Cel-Sci shares were trading at $1.73 a share Wednesday morning.
The Vienna, Va.-based company said its latest application covers its drug CEL-1000 for both the prevention and treatment of bird flu. The drug provides broad-spectrum protection, which Cel-Sci said is important against the bird flu virus because the virus exhibits a high mutation rate.
In December, Cel-Sci, which maintains a research and development facility in Baltimore, signed an agreement with the National Institutes of Health to test the vaccine as a potential Avian flu vaccine in clinical trials with animals. The drug has shown positive results in animal tests against herpes simplex virus, encephalitis and malaria.
Cel-Sci (AMEX: CVM) is one of several area biotechs developing an Avian flu vaccine. Gaithersburg-based MedImmune (NASDAQ: MEDI) is also working with NIH to produce and test vaccines against potential pandemic flu strains.
Novavax (NASDAQ: NVAX) is advancing clinical trials of its experimental bird flu vaccine. The company is now based in Philadelphia, but has a research facility in Rockville.
Cel-Sci's stock has doubled since the beginning of the year.
|
why prepare & stockpile an anti-viral remedy for a virus
that hasn't even mutated to Human to Human form yet?
it will be rendered useless to kill any new strain...
these anti-virals might not be
for the treatment of 'avian' flu at all
but for the propogation of a cash cow
ask your doctor about the dangers of
self prescribing anti viral medication
your body builds up a resistance to it
and the virus does too...thus it mutates
my hunch is that the mass marketing & selling of
Tamiflu by use of viral marketing and threataganda
will help the mutation of the virus
into yet another money spinner
it is a beast that feeds itself
|
Bird flu vaccine developed
11-April 2003
"A team of researchers - led by Dr Richard Webby, who graduated from Otago in 1999, and a colleague, both based at St Jude Children's Research Hospital in Memphis, America - has rapidly developed a vaccine for a deadly Hong Kong bird virus and human killer."
"Using samples of the influenza from Hong Kong, they mixed two genes from the virus with six genes from another virus inside a cell. This modified the virus genes to "abolish its ability to cause disease and therefore made it safer to use as a vaccine", the statement said. The virus has been sent to Atlanta and London for testing in preparation for human trials."
Otago graduates help create flu vaccine- Joanna Norris
Otago Daily Times
|
OOPS! : Vaccine helps to mutate Bird flu?
11 February 04
..."In 2003, scientists who developed an improved flu vaccine for poultry, including Robert Webster of St Jude's, concluded that such vaccination "may be a serious problem for human pandemic preparedness" (Virology, vol 314, p 580).
Such vaccines, they wrote, might mask disease signs while allowing the birds to continue to shed virus. In such a case, "persistence of virus infection in the presence of a flock immunity may contribute to increased virus evolution".
Genetic analysis probes bird flu's history - New scientist
|
|
According to the CDC article H5N1 Outbreaks and Enzootic Influenza by Robert G. Webster et. al.:
"Transmission of highly pathogenic H5N1 from domestic poultry back to migratory waterfowl in western China has increased the geographic spread. The spread of H5N1 and its likely reintroduction to domestic poultry increase the need for good agricultural vaccines. In fact, the root cause of the continuing H5N1 pandemic threat may be the way the pathogenicity of H5N1 viruses is masked by cocirculating influenza viruses or bad agricultural vaccines." - [cdc.gov]
Dr. Robert Webster explains: "If you use a good vaccine you can prevent the transmission within poultry and to humans. But if they have been using vaccines now [in China] for several years, why is there so much bird flu? There is bad vaccine that stops the disease in the bird but the bird goes on pooping out virus and maintaining it and changing it. And I think this is what is going on in China. It has to be. Either there is not enough vaccine being used or there is substandard vaccine being used. Probably both. It’s not just China. We can’t blame China for substandard vaccines. I think there are substandard vaccines for influenza in poultry all over the world." - [Expert: Bad vaccines may trigger China bird flu]
In response to the same concerns, Reuters reports Hong Kong infectious disease expert Lo Wing-lok saying, "The issue of vaccines has to take top priority," and Julie Hall, in charge of the WHO's outbreak response in China, saying China's vaccinations might be masking the virus." - [China H5N1 outbreak puts vaccines under spotlight]
The BBC reported that Dr Wendy Barclay, a virologist at the University of Reading, UK said: "The Chinese have made a vaccine based on reverse genetics made with H5N1 antigens, and they have been using it. There has been a lot of criticism of what they have done, because they have protected their chickens against death from this virus but the chickens still get infected; and then you get drift - the virus mutates in response to the antibodies - and now we have a situation where we have five or six 'flavours' of H5N1 out there."- [Bird flu vaccine no 'silver bullet' ]
- wikipedia.org/
|
|
Scientists warn of possibility of drug-resistant avian flu
By Justin Teo, RSI First published 11 October 2005
US scientists warn that a drug-resistant Avian flu strain could arise with unrestrained and improper use of available drugs. The warning came as governments around the world are stockpiling antiviral drugs and the H5N1 avian flu strain threatens to break out into a flu pandemic.
The virus has killed 65 people in Asia since late 2003 and recent reports of an outbreak amongst poultry in Turkey are unnerving European states. How could improper or unrestrained use of antiviral drugs affect the battle against Avian flu?
Justin Teo spoke to Dr. Jeffrey Staples, Senior Medical Advisor at International SOS, for more.
JS: Well, there are actually two ways that it could affect that I can see. Firstly, the anti-viral drugs are already in limited supply, so improper or unrestrained use of them could already reduce the supply significantly. Also there's the issue of drug resistance to develop and as proven with other anti-viral medication and bacteria, the unrestrained use of anti-viral drugs can lead to drug resistance. If the avian flu does become pandemic, then these two anti-viral drugs will be effective against avian flu to an extent. The concern is that people will be using the medication improperly, maybe when they don't have the virus or maybe they'll be using it improperly when they do and some resistance could develop. I think that it's safe to say that the anti-viral drugs will continue to be effective but if resistance develops, that resistance is likely to decrease.
JT: If resistance against the virus does decrease, and in the case of Vietnam where a patient did not respond to the drug Tamiflu, would a higher dosage work?
JS: It's certainly possible although I've not seen any studies out there with conclusive evidence but it's certainly possible that a higher dosage or a longer treatment course could be effective. Now unfortunately if this is the case, it'll put a further strain on the supplies of the medication.
JT: Michael Osterholm, an infectious disease expert advising the US government, mentioned that if the H5N1 bird flu virus infects humans, it will move too quickly for drugs and vaccines to be of much use. How true is this?
JS: I think he is absolutely correct in a lot of ways. First and foremost, on the vaccine front, there is no vaccine against the avian flu virus. The US has developed one in experimental stages, but that's just a vaccine out there currently in birds. If this virus mutates into a human pandemic form, we'll not have a vaccine for that virus probably for six to nine months because we don't know what the virus looks like yet and so we can't make a vaccine for it. In terms of the medication, again, the supplies of the medication are limited and the distribution of the medication is probably going to lag behind the spread of the virus, even in our best efforts. In the current phase, where the virus seems to be having great difficulty infecting humans, we are probably well able to catch up and contain it. But if it mutates into a full pandemic form, it will probably spread beyond our capabilities to contain it with medication.
JT: All this reflects the world's reliance on vaccines and drugs to combat the avian flu. What alternatives are there to fight the virus?
JS: I think the world in general is looking for a sure thing, quick-easy fix, in terms of vaccine and medication. Where there certainly is a reasonable component of a preparedness pandemic plan, they are not going to be in of itself be able to stop the pandemic should the pandemic occur. So alternatives are really good, comprehensive planning for infrastructure, communications, transportation, logistics and supplies, and planning alternative sources and alternative means of getting critical operations done. We basically have to figure out how we can operate our society should a pandemic occur and we shouldn't just rely on vaccines and drugs. - channel news asia
|
|
WHO mulls centralizing flu vaccine
Monday 31st October, 2005 (UPI) - The World Health Organization is looking into creating a centralized system to purchase flu vaccines designed to reduce costs and boost immunization rates.
Dr. Klaus Stohr, coordinator of the WHO global influenza program, told the Financial Times by purchasing the seasonal flu vaccines in bulk on behalf of groups of countries working together, the organization could negotiate lower prices, making seasonal vaccines more attractive and retaining some of the price difference to invest in research and development.
However, one representative of the vaccine manufacturers cautioned that a similar scheme operated by UNICEF drove down vaccine prices so far that producers withdrew and investment in innovation was reduced.
Jean-Pierre Garnier, GlaxoSmithKline's chief executive called on U.S. regulators to allow the leading vaccine companies to cooperate, despite anti-trust concerns, so that they could all produce the single pandemic vaccine which proved the most effective in clinical trials.
- Big News Network.com
|
guesswork?
U.S. to create a bird flu virus mutation
Atlanta, GA, Mar. 24 (UPI) -- The U.S. Centers for Disease Control and Prevention has begun a series of experiments to see how likely the bird flu virus could result in a human pandemic.
The six-month series of experiments seeks to simulate the mixing and matching of genes from the H5N1 avian flu virus that has plagued Asia and a common human flu virus that public-health experts fear could turn avian flu into a pandemic, the Wall Street Journal reported Thursday.
CDC scientists inside an ultra-secure laboratory have started swapping the genes of the H5N1 avian virus with the genes of an H3N2 virus, the strain behind most recent human flu outbreaks.
The goal is to substitute the eight genes of each virus, one by one, with the eight genes from the other virus to see which of more than 250 possible combinations create flu viruses that could spread easily among humans.
The work responds to fears by global public health experts that the bird flu virus could mutate to form one that could spawn a global outbreak of the disease. - washingtontimes.com
|
Round & round it goes
Influenza viruses continually mutate or change, which enables the virus to evade
the immune system.
People are susceptible to influenza infection throughout their lives.
The process works as follows:
A person infected with influenza virus develops antibody against that virus.
The virus mutates or changes.
The "older" antibody no longer recognizes the "newer" virus.
Reinfection occurs.
The older antibody can, however, provide partial protection against reinfection.
Currently, three different influenza strains circulate worldwide:
two type A viruses and one type B. Type A viruses are divided into subtypes based
on differences in two viral proteins called hemagglutinin (H) and neuraminidase (N).
The current subtypes of influenza A are designated A(H1N1), A(H3N2),
and B(Hong Kong/330/2001-like virus strain).
source
|
|
Of course - the timeframe for these Avian flu scares - right through from summer 2005 into early 2006... are purely co-incidental to yet another push by London & Washington to 'bring Iran & Syria into line'
Bird Flu means imposing a kind of martial law, making home grown food & local farms feel untrustworthy - keeping the public scared witless....
Coalition forces are readying the spin machine to enable bombing the shit out of The axis of evil
|
|
politically useful?
Is Avian Flu another Pentagon Hoax?
F. William Engdahl
October 30, 2005
No sooner are indictments being handed down to Scooter Libby, the Chief of Staff of the Vice President of the United States for lies and coverup of information used deliberately to suppress the fact the Bush Administration had no 'smoking gun' to prove Saddam Hussein was building a nuclear arsenal, but a new scandal is surfacing every bit as outrageous and ultimately, likely also criminal.
Against all scientific prudence and normal public health procedure, the world population is being whipped up into a fear frenzy by irresponsible public health officials from the US Administration to WHO to the United States Centers for Disease Control. They all warn about the imminent danger that a malicious viral strain might spread from infected birds, primarily in Vietnam and other Asian centers, to contaminate the entire human species in pandemic proportions. Often the flu pandemic of 1918 which is said to have killed 18 million worldwide, is cited as an example of what 'might' lie in store for us.
On November 1, appropriately enough the day after Halloween, President Bush is scheduled to visit the National Institutes of Health in Bethesda Maryland to announce his Administration's strategy of how it will prepare for the next flu epidemic, whether from Bird Flu or some other strain. The plan has been a year in the making. On October 28 the Senate passed an $8 billion emergency funding bill to address the growing Avian Flu panic. Health and Human Services Secretary Mike Leavitt, in a moment of candor during the debate on the Senate bill told the press, 'If it isn't the current H5N1 virus that leads to an influenza pandemic, at some point in our nation's future, another virus will.' In the meantime taxpayer billions will have gone to a handful of pharmaceutical giants positioned to profit. None stands to reap more lucre than the Swiss-US pharmaceutical giant Roche Holdings of Basle.
The only medicine we are told which reduce the symptoms of general or seasonal influenza and 'possibly' might reduce symptoms also of Avian Flu, is a drug called Tamiflu. Today the giant Swiss pharmaceutical firm, Roche, holds the sole license to manufacture Tamiflu. Due to the media panic, the order books at Roche today are filled to overflowing. Roche recently refused a request from the US Congress to lift its exclusive patent rights to allow other drug manug´facturers to produce Tamiflu with the improbable excuse that it was in effect, too complex for others to rapidly produce.
However, the real point of interest is the company in California who developed Tamiflu and gave the marketing rights to its patented discovery to Roche.
'Rummy Flu'
Tamiflu was developed and patented in 1996 by a California biotech firm, Gilead Sciences Inc. Gilead is a NASDAQ (GILD) listed stock company which prefers to maintain a low profile in the current rush to Tamiflu. That might be because of who is tied to Gilead. In 1997, before he became US Secretary of Defense, Donald H. Rumsfeld was named Chairman of the Board of Gilead Sciences, where he remained until early 2001 when he became Defense Secretary. Rumsfeld had been on the board of Gilead since 1988 according to a January 3 1997 company press release.
An as-yet-unconfirmed report is that Rumsfeld while Secretary of Defense also purchased an additional stock in his former company, Gilead Sciences Inc., worth $18 million, making him one of its largest if not the largest stock owners today.
The Secretary of Defense, the man who allegedly supported the use of contrived intelligence to justify the war on Iraq, is now poised to reap huge gains for a flu panic his Administration has done everything it can to promote. It would be useful to know whether the Pentagon's successor to Douglas Feith's Office of Special Plans developed the strategy of biowarfare behind the current Avian Flu panic. Perhaps some enterprising Congressional committee might look into the entire subject of plausible conflicts of interest regarding Secretary Rumsfeld.
Rumsfeld stands to make a fortune on royalties as a panicked world population scrambles to buy a drug worthless in curing effects of alleged Avian Flu. The model suggests the parallel to the brazen corruption of Halliburton Corporation whose former CEO is Vice President Dick Cheney. Cheney's company has so far gotten billions worth of US construction contracts in Iraq and elsewhere. Coincidence that Cheney's closest political friend is Defense Secretary and Avian Flu beneficiary Don Rumsfeld? It is another example of what someone has called the principle of modern US corrupt special interest politics: 'Concentrate the benefits; diffuse the costs' President Bush has ordered the US Government to buy $2 billion worth of Gilead Science's Tamilflu.
GMO Chickens come home to roost
But Tamiflu conflicts are perhaps just the tip of the iceberg of the Avian Flu story. There is high-level biological research underway in Britain and presumably also the United States to develop a genetic engineering method to make chickens and other birds 'resistant' to Avian Flu viruses.
British scientists are reportedly genetically engineering chickens to produce birds resistant to the lethal strains of the H5N1 virus devastating poultry in the Far East. Laurence Tiley, Professor of Microular Virology at Cambridge University and Helen Sang of the Roslin Institute in Scotland are involved in developing 'transgenic chickens' which would have small pieces of genetic material inserted into chicken eggs to allegedly make the chickens H5N1 resistant.
Tiley told the Times of London on October 29, 'Once we have regulatory approval, we believe it will only take between four and five years to breed enough chickens to replace the entire world (chicken) population.' The real question in this dubious undertaking is which GMO giants are underwriting the research and development of GMO chickens and who will control their products. It is increasingly clear that the entire saga of Avian Flu is one whose dimensions are only slowly coming to light. What we can see so far is not at all pretty.
- William Engdahl
|
politically useful?
War Against Iran, April 2006
Biological Threat and Executive Order 13292
by Jorge Hirsch [antiwar.com]
H istory repeats itself, but always with new twists.
We are back to the good old days when a
Declaration of War preceded the start of a war. Such declaration
occurred on March 16th, 2006. Reversing the old order, we are now in the
"Sitzkrieg",
to be followed shortly by an aerial
"Blitzkrieg" in the coming days.
In the old days, Congress declared war, and
directed the Executive to take action. In the new millenium,
the Executive declared war last March 16th, then Congress will pass
H.R. 282, "To hold the current regime in Iran accountable for its threatening
behavior and to support a transition to democracy in Iran." This bill and
previous ones like it are in direct violation of the legally binding
Algiers Accords[pdf] signed by the United States and Iran on January 19,
1981, that states "The United States pledges that it is and from now on
will be the policy of the United States not to intervene, directly or indirectly,
politically or militarily, in Iran's internal affairs"; however, this is
clearly of
no interest to the
353 policymakers sponsoring the bill.
The US promised Russia and China that
the UN Security Council statement just approved will not be a trigger for
military action after 30 days; true to its promise, the US will attack before
the 30-day deadline imposed by the UNSC for Iran to stop its nuclear enrichment
activity, i.e. before the end of April. The "justification" is likely to be
an alleged threat of
imminent biological attack with Iran's involvement.
The Declaration of War against Iran
I n the aftermath of Pearl Harbor, the
Congressional Declaration of December 8, 1941 stated: " Whereas the Imperial
Government of Japan has committed unprovoked acts of war against the Government
and the people of the United States of America: Therefore be it Resolved by
the Senate and House of Representatives of the United States of America in Congress
assembled, That the state of war between the United States and the Imperial
Government of Japan which has thus been thrust upon the United States is hereby
formally declared; and the president is hereby authorized and directed to employ
the entire naval and military forces of the United States and the resources
of the Government to carry on war against the Imperial Government of Japan."
Similarly, the formal war declaration against Iran, the
National Security Strategy of March 16, 2006, stated:
- "We may face no greater challenge from a single country than from Iran."
- "The Iranian regime sponsors terrorism; threatens Israel; seeks to thwart
Middle East peace; disrupts democracy in Iraq; and denies the aspirations
of its people for freedom."
- "[T]he first duty of the United States Government remains what it always
has been: to protect the American people and American interests. It is an
enduring American principle that this duty obligates the government to anticipate
and counter threats, using all elements of national power, before the threats
can do grave damage."
- "The greater the threat, the greater is the risk of inaction and the
more compelling the case for taking anticipatory action to defend ourselves,
even if uncertainty remains as to the time and place of the enemy's attack.
There are few greater threats than a terrorist attack with WMD."
- "To forestall or prevent such hostile acts by our adversaries, the United
States will, if necessary, act preemptively."
- "When the consequences of an attack with WMD are potentially so devastating,
we cannot afford to stand idly by as grave dangers materialize."
- "[T]here will always be some uncertainty about the status of hidden programs."
- "Advances in biotechnology provide greater opportunities for state and
non-state actors to obtain dangerous pathogens and equipment."
- "Biological weapons also pose a grave WMD threat because of the risks of
contagion that would spread disease across large populations and around the
globe."
- "Countering the spread of biological weapons .... will also enhance our
Nation's ability to respond to pandemic public health threats, such as avian
influenza."
This has to be combined with the 2005 U.S. State Department "FINDING.
The United States judges that, based on all available information, Iran has
an offensive biological weapons program in violation of the BWC."
In addition, the March 16 declaration makes it clear that the US
will use nuclear weapons in the war against Iran:
- ."..using all elements of national power..."
- "Safe, credible, and reliable nuclear forces continue to play a critical
role. We are strengthening deterrence by developing a New Triad composed of
offensive strike systems (both nuclear and improved conventional capabilities)."
and this is further reinforced by the just
released
"National Military Strategy to Combat Weapons of Mass Destruction"[pdf]
that states "Offensive operations may include kinetic (both conventional and
nuclear) and/or non-kinetic options (e.g. information operations) to deter or
defeat a WMD threat or subsequent use of WMD."
There is of course also the
claim that Iran is a threat because it intends to develop nuclear weapons.
The sole purpose of that claim, which
flies in the face of all available evidence, is to
generate a diplomatic stalemate at the UN that will allow Bush to state
that
other nations share the US concern but not the resolve to act. However the
actual trigger for the bombing to begin will not be the
long-term and
by now discredited nuclear threat, rather it is likely to be the
threat of an imminent biological attack.
Casus Belli
There is no casus belli against Iran based on
its nuclear program. The
IAEA has found no evidence that in the 20 years of its development there
has been any diversion of nuclear material to military applications. The Bush
administration now
officially acknowledges that the issue with Iran arises from a
"loophole" in the Nuclear Non-Proliferation Treaty, that allows non-nuclear
countries to pursue uranium enrichment.
However it is not a loophole, the right to a full civilian nuclear program is
an integral part of the compromise, that made non-nuclear countries agree
to it. For the US to call it a loophole means to abrogate the treaty unilaterally
and propose a different treaty that non-nuclear countries will have no motivation
to agree to.
The Bush administration declares that a civilian nuclear program that gives
Iran
"knowledge" or
"capability" to build a nuclear weapon is unacceptable. It could apply exactly
the same logic to biotechnology.
The State Department says that "Iran is expanding its biotechnology and
biomedical industries by building large, state-of-the-art research and pharmaceutical
production facilities. These industries could easily hide pilot to industrial-scale
production capabilities for a potential BW program, and could mask procurement
of BW-related process equipment." Why isn't the US demanding that Iran stops
its biotechnology research and development, and that
it transfers all biotech related activities to Russia?
The key lies in
Executive Order 13292, which made information on "weapons of mass destruction"
and on "defense against transnational terrorism" classified. If concrete details
about Iran's
alleged biological weapons programs were made public, they would be subject
to public scrutiny and they would be discredited, as the
allegations on Iran's "nuclear weapons program" have been. The US is likely
to have "assembled" classified information on Iran's biological weapons programs
and shared it with selected individuals, including members of Congress, under
the constraint that classified information cannot be made public. For example,
at the
June 25, 2004 House subcommittee "MEMBERS ONLY CLASSIFIED BRIEFING on Iran,
Middle East Proliferation and Terrorist Capabilities." The unclassified
portion of that
briefing states "It is time for Iran to declare its biological weapons
program and make arrangements for its dismantlement."
There is likely to be a
team of "experts" lined up by the administration that will support its
claims that Iran had a biological weapons program representing an imminent
threat. There is always room in science for differing opinions, and if an open
scientific debate is not possible because information is classified, any outlandish
claim can find some supporters in the scientific community. The
most likely biological threat to be invoked, because it has a
natural time element associated with it, is the
threat of a bird flu pandemic caused by a
deliberately mutated H5N1 virus carried
by migrating wild
birds.
The
Biological Threat
C onsider for example
Dr. Ward Casscells, a renowned cardiologist that has of late
become an "expert" in bioterrorism. Even more recently, Dr. Casscells
joined the Army as a colonel . According to the US Defense Department,
"his years of research on now-spreading avian flu are now deemed cutting edge."
However, I know of no independent credible scientific body that makes the same
assessment: Dr. Casscells has written a total of four papers on the effect of
influenza on cardiac disease which have been cited by no other scientists. His
paper "Influenza as a bioweapon" has a grand total of 5 citations, meaning a
mere 5 other papers refer to it; "cutting edge" scientific papers have hundreds
or thousands of citations. His only other paper on the subject, "Influenza as
a bioterror threat: the need for global vaccination" has zero citations.
Nonetheless,
Dr. Casscells' outstanding credentials as a scientist will be invoked by
the administration if he vouches for the credibility of "intelligence" indicating
that a dangerous mutated bird flu virus has been developed in an Iranian underground
bioweapons laboratory. Dr. Casscells has been surveilling the Middle East to
"scope out the possibility for a widespread outbreak" of bird flu. Because
he has been advocating the view that
"Bird flu is poised to be an explosive problem" and has
predicted the use of influenza as a bioweapon, he is likely to be inclined
to believe such claims. Similarly his scientific colleagues at the
"Defense of Houston" committee, that work on anticipating bioterrorism threats
and
are highly lauded by the administration and very well
funded by Army grants.
The
Bush administration has spent
vast sums of money in combating bioterrorism threats, reportedly
over $7 billion per year, without any evidence or precedent for bioterrorism
attacks. Nevertheless
there will always be plenty of scientists that will flock to
where the grant money is and devote efforts to validate conclusions that
are valued by the organizations giving the grants,
and news media duly publicize the
hyped threat of bioterrorism. Still, last year over
700 scientists including 2 Nobel laureates signed a petition objecting to
the diversion of funds from projects of high public-health importance to biodefense,
calling it a "misdirection" of priorities. Dr. Richard H. Ebright, a renowned
molecular biologist,
states that "A majority of the nation's top microbiologists the very group
that the Bush administration is counting on to carry out its biodefense research
agenda dispute the premises and implementation of the biodefense spending."
On the supposed threat of bird flu, while it is continuously being hyped by
the administration
[1],
[2],
[3],
[4],
[5], expert opinion is that it is not a serious threat
[1],
[2],
[3],
[4],
[5],
[6]
and is politically motivated. The blaming of bird flu spread on wild birds
is also highly questionable [1],
[2].
On March 15th, right before the disclosure of the new National Security Strategy,
I suggested
the bird flu casus belli against Iran, that would "necessitate" bombing
of Iranian facilities before the bird migration season begins in the
Spring. Several elements emphasized in the March 16 NSS appear to support that
scenario, as
discussed above. In a
March 20 press conference concerning federal preparedness for avian flu,
Secretary Michael Leavitt (who also
warned a few weeks ago to store tuna and milk under the bed to prepare for bird
flu ) stated "Think of the world if you will as a vast forest that is susceptible
to fire. A spark if allowed to burn will emerge as an uncontainable fire. That's
a pandemic. If we are there when the spark happens, it can be squelched. But
if allowed to burn for a time it begins to spread uncontrollably." An aerial
attack on Iranian installations may be touted as the "squelching" of the bird
flu pandemic spark.
Does Bush need congressional authorization to bomb Iran?
The answer is contained in the
Statement by the president of October 16, 2002, in signing into law the
congressional authorization to use force against Iraq. It states
"...I sought an additional resolution of support from the Congress to
use force against Iraq, should force become necessary. While I appreciate receiving
that support, my request for it did not, and my signing this resolution does
not, constitute any change in the long-standing positions of the executive branch
on either the president's constitutional authority to use force to deter, prevent,
or respond to aggression or other threats to U.S. interests or on the constitutionality
of the War Powers Resolution."
In other words: "I appreciate Congress' authorization but didn't need it and
will not need it next time with Iran."
The
War Powers Resolution encourages the president to consult with Congress
"in every possible instance",
yet allows the president to introduce Armed Forces into hostilities without
Congressional authorization; it simply compels him to terminate hostilities
within 60 to 90 days unless Congress authorizes an extension. Plenty time enough.
The Attack
I t is unlikely that there will be a public announcement
of the impending attack before it starts, since it would generate opposition.
Allies
do not want to be implicated and will deny any knowledge. Who will be officially
notified that an attack is about to take place? Most likely, Iran itself.
Direct conversations between the US and Iran are about to start, nominally
on the subject of Iraq only. They will also provide the only direct conduit
for the US to communicate with Iran without intermediaries. An "ultimatum" unacceptable
to Iran, as was delivered publicly
to Iraq on March 17th, 2003, could be delivered privately to Iran through
that route.
The reasons for our actions will be clear, the force measured, and the
cause just.
The initial US attack on Iranian facilities is likely to be "measured": a
highly accurate strike on selected facilities "suspected" of bioweapons work,
with
cruise missiles launched from
submarines or ships in the Persian Gulf. That is a component of the
CONPLAN 8022 Global Strike mission, which
recently became operational and also includes
nuclear preemptive strikes.
The "clear" reasons and "just" cause for the administration to attack can
be stated as follows: if
a bird flu pandemic can cause 150 million deaths and there is even a one
percent probability that the
"intelligence" is right, i.e. even if there is a 99%
"uncertainty about the status of hidden programs", the
expected number of deaths that would be prevented by bombing the Iranian
facilities is the product of those two numbers, i.e. 1.5 million, vastly larger
than the few thousand Iranian casualties due to "collateral damage."
Any
military
reaction by Iran to the attack, perhaps even a verbal reaction, will be
construed as
"aggression" by Iran towards the US and Israel, and result in large scale
bombing of Iranian missile, nuclear and other facilities. Does that sound absurd?
Recall that
the US and
Britain bombed
Iraq's no-fly zones well before the Iraq invasion, and Iraqi response was
labeled
"aggression toward planes of the coalition forces."
Nuclear
earth penetrating weapons may be used in the initial attack, and
certainly will be used in the large scale attack that will follow.
Why will this happen? Because it was
"pencilled in" a long time ago. The
actions of the US against Iran in
recent years have been clearly directed towards a confrontation, to
suppress the rise of Iran as a strong regional power that does not conform
to US interests.
Can it be Prevented?
A
small group of thugs is about to lead America across
a line of no return. On the other side of this line there is no nuclear
taboo, no restraint on preemptive nuclear attacks on non-nuclear nations, and
no incentive for non-nuclear nations to remain non-nuclear. A global nuclear
war and the destruction of humanity will be a distinct possibility.
Americans are largely
unaware of what is about to happen.
Half a million people go to the streets on immigration law, yet nobody is
demonstrating against the Iran war that will radically change the life of Americans
for generations to come. The more informed sectors of society, scientists, arms
control organizations, the media, the political establishment, the military,
are not taking a strong stand against the impending war.
Congress is silent.
Only people in the know can stop this. Resigning from the job is not good
enough
[1],
[2],
[3]. People in the know
have to come forward with information that brings the impending attack to
the forefront of attention of Congress and the American public and thwarts it.
Not doing so is being complicit in a plan that will bring tragic consequences
to America and the world.
Else, all that will be left is to
bring the perpetrators to justice. Danton, Robespierre, Mussolini, Petain,
Ribbentrop, Goering, Ceausescu also occupied positions of power and prominence
at some point in their careers.
|
|


