Delta Variant panic globally
|
Update on the global situation
|
THE PCR VACCINE MERRY GO ROUND? Swab humanitys DNA Use PCR to make RNA into cDna (legally patentable) as a copy Dna database scan using AI to find variants Use CT PCR to extract cRna from variant sample Place cRna variant sample in Mrna gene therapy (now labelled as vaccine) |
|
|
|
|
now please take a look at this
|
"PCR can generate 100 billion similar molecules in an afternoon."
Kary B. Mullis: The Unusual Origin of the Polymerase Chain Reaction (Scientific American April 1990)
GENERATE
it allows for the unlimited replication of small bits of DNa
HOW DIFFERENT TO THIS IS CLONING?
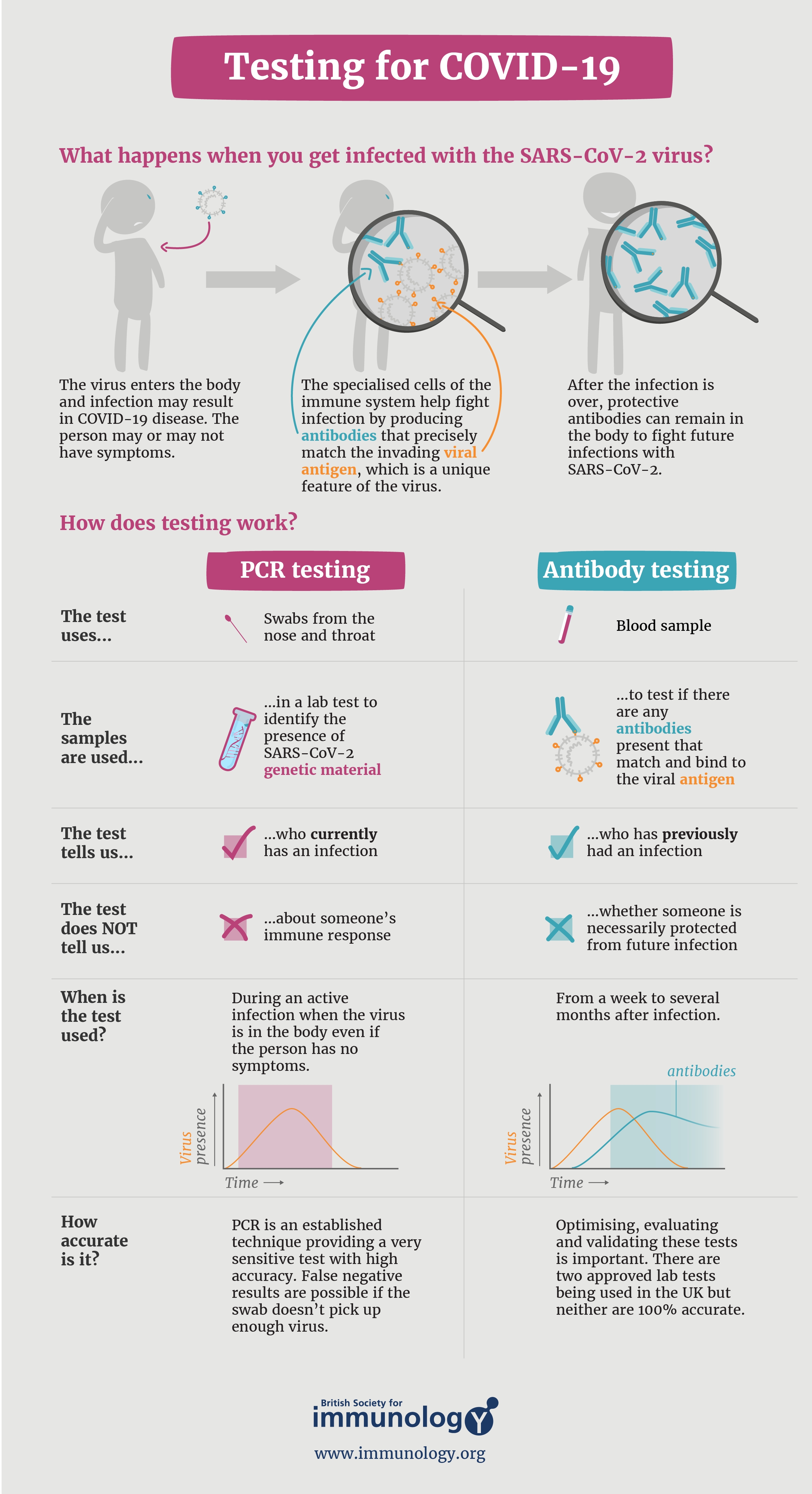
what has PCR testing done?
replicated a, upon all evidence available, a reletavily harmless, Novel coronavirus disease,
where is the DNA material disposed?
|

|
what happens to the replicated DNA material from everyone that's tested?
Are Humans being swabbed on mass for DNA for a reason?
is there a specific DNA being looked for?
For Military bioweapons R&D?
PCR inventor Kary Mullis is credited with making the human genome project possible
I fear a genetic library of humanity is being assembled
and from that library any one can be cloned.
humanity produced like off the shelf consumerables
|
|
"...is the drive for COVID-19 testing just a smokescreen with regard to the PCR test? The answer to this simple question is a straightforward yes, it is. In reality, the PCR tests have another much more important role, and that is sampling DNA data to facilitate genome sequencing, the name for reading the genetic code for the makeup of the human body."
click on pic for article/ video
|
|
did NOT come from an
isolated virus or an infected person
|
What's really going on?

With regard to RT-PCR assays, given that all coronaviruses have an RNA genome, it is necessary to synthesize complementary DNA (cDNA) from the RNA genome through reverse transcription, followed by PCR amplification of the cDNA with specific primers for the SARS-CoV-2 genes of interest. While all NAATs that utilize RT-PCR detect SARS-CoV-2 in this way, there are many variations that can be applied for the actual detection of the amplified genes. Most common is the use of a real-time RT-PCR, which employs fluorescence to detect the amount of amplified DNA in real time. A frequently utilized example of this is TaqMan hydrolysis. [38] In real-time RT-PCR, the amount of gene target present in the sample typically determines the number of PCR cycles (known as the cycle threshold [Ct] value) needed before SARS-CoV-2 is detected. Medscape |
cDNA WAS RULED PATENTABLE
see page 3
and
Nature article here
GUESS WHAT PCR TESTS GENERATE FROM human swabbed source RNA?
cDNA
classed as not 'human' & Patentable.
this is precisely why PcR should not be used in diagnostic situations
so a human dna database collected from swabed PCR tests
would contain patented cDNA generated from from the human population
sound a bit dodgy?
yup
not as dodgy as generating new variants from
the cDna collected
and making a Mrna gene therapy into a
politically useful bioweapon
|
|
Reverse transcriptases (RTs) are RNA-directed DNA polymerases that were first identified as part of the retroviral life cycle (Temin and Mizutani, 1970, Baltimore, 1970). RTs catalyze the synthesis of a DNA copy (cDNA) of the target RNA molecules using a reverse transcription primer, dNTPs, and Mg2+ or Mn2+ as a cofactor. Reverse transcriptases have been adapted for use in a variety of in vitro applications including real-time and endpoint RT-PCR, labeled-cDNA probe generation and cDNA library construction. |
|
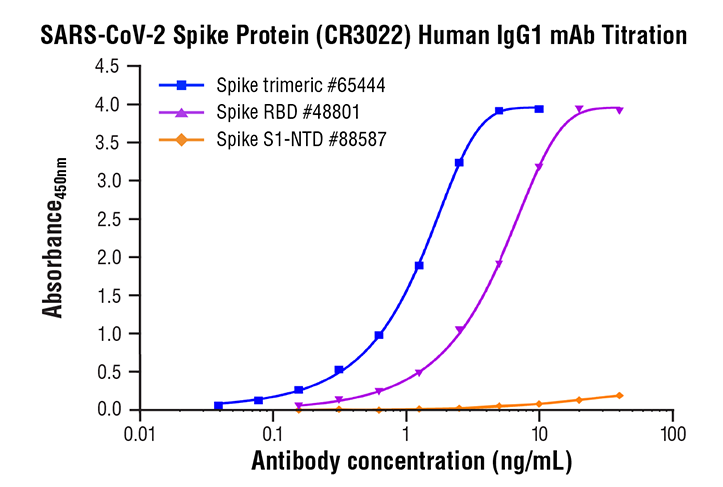
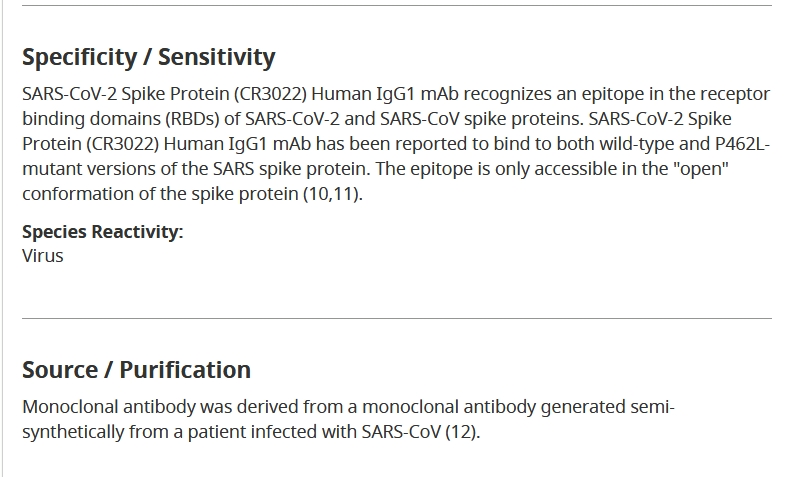
Several mAbs have shown promising results in neutralizing SARS-CoV-2.
CR3022, a SARS-CoV-specific human mAb, binds potently with SARS-CoV-2 (KD of 6.3?nM, measured by BLI in OctetRED96), suggesting that CR3022 has the potential to be developed as candidate therapeutic, alone or in combination with other nAbs, for the prevention and treatment of SARS-CoV-2 infection [57].
A mAb targeting S1 prepared from immunized transgenic mice expressing human Ig variable heavy and light chains has recently been shown to neutralize both SARS-CoV-2 and SARS-CoV infections via an unknown mechanism that is independent of the blockade of RBD–hACE2 interaction [58].
Recently, many human blocking mAbs (311mab-31B5, 311mab-32D4, 47D11, n3130, n3088, S309, P2C-1F11, P2B-2F6, B38, H4)
have been successfully cloned from single memory B cells from recovered COVID-19 patients [58,59,60,61,62,63].
These mAbs specifically bind to SARS-CoV-2 S to effectively neutralize infection.
In addition, sera from SARS patients during rehabilitation or animals specifically immunized with SARS-CoV S1 may cross-neutralize SARS-CoV-2 and reduce S protein-mediated SARS-CoV-2 entry
|
|
|
Protein Engineering for Therapeutics, Part A
Saravanan Rajan, Sachdev S. Sidhu, in Methods in Enzymology, 2012
Abstract
Synthetic antibody libraries are constructed from scratch using designed synthetic DNA.
Precise control over design enables the use of highly optimized human frameworks and the introduction of defined chemical diversity at positions that are most likely to contribute to antigen recognition.
We describe complete methods for the design, construction, and application of simplified synthetic antibody libraries built on a single human framework with diversity restricted to four complementarity-determining regions and two amino acids (tyrosine and serine).
Despite the extreme simplicity of design, these libraries are capable of generating specific antibodies against diverse protein antigens. Moreover, the same methods can be used to build more complex libraries that can produce synthetic antibodies with affinities and specificities beyond the scope of natural antibodies.
Most importantly, these simplified methods rely on standard supplies, equipment, and methods that are accessible to any molecular biology laboratory.
Science direct
|
|
EcoHealth Alliance Announces Discovery of SARS-Like Viruses Spilling Over into People in China
NEW YORK – March 2, 2018 – The result of its continued surveillance of people and animals in emerging disease hotspot regions, EcoHealth Alliance, a nonprofit organization working at the intersection of animal, environmental, and human health on a global scale, has found evidence of viruses closely related to SARS in people in Yunnan Province, China. Published today in Virologica Sinica, this research confirms that coronaviruses capable of infecting humans continue to lurk in the wild. It also confirms the importance of continued study of these unknown viruses before they create outbreaks like the 2002-2003 SARS pandemic.
"We want to be clear that this doesn’t necessarily indicate a brewing outbreak," paper co-author and EcoHealth Alliance President Dr. Peter Daszak said. "It does, however, quite clearly show the value of continued biosurveillance in hotspot regions like Southeast Asia. If we know what viruses are out there in wildlife, and which people are getting infected, we have a chance to stop pandemics dead in their tracks."
EcoHealth Alliance scientists, in partnership with Wuhan Institute of Virology and Duke-NUS, found SARSr-CoVs in bat caves near Jinning in Yunnan Province. Human testing revealed antibodies against SARSr Co-V in six people living nearby, though none recalled any symptoms related to SARS infection.
In 2005, EcoHealth Alliance scientists were among those invited by Chinese virologists and public health scientists to investigate the origins of the 2002-2003 SARS pandemic. They concluded that bats are the reservoir species for SARSr-CoVs, and it was these viruses that infected people and civets being sold for consumption in live animal markets, leading to the SARS outbreak. The findings published today provide early evidence that conditions exist for SARS spillover in other areas outside Guangdong as well.
These findings come as the World Health Organization updated its R&D Blueprint list of priority pathogens, i.e. those that should be prioritized for global research. The list includes coronaviruses such as SARS and MERS (Middle East respiratory syndrome). It also includes Disease X, which represents the global health community’s understanding that the next pandemic could likely come from a pathogen currently unknown to infect humans. There are currently an estimated 1.67 million unknown viruses on Earth.
These findings highlight the importance of EcoHealth Alliance’s global biosurveillance work. Scientists and local partners are currently testing human and animal specimens for known and unknown pathogens in over 15 countries in an attempt to stop the next pandemic before it begins.
|

|
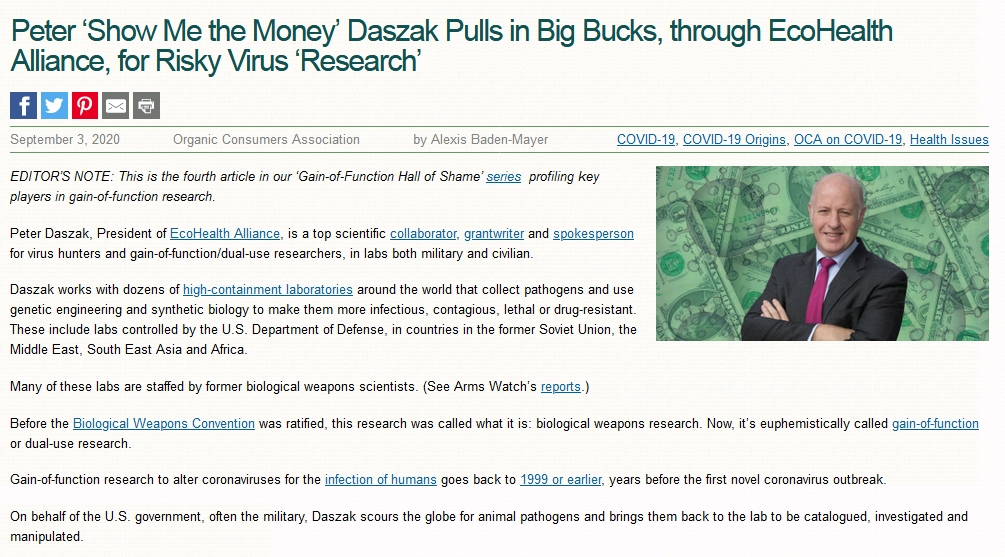
Dr. Peter Daszak
is President of EcoHealth Alliance, a US-based organization that conducts research and outreach programs on global health, conservation and international development. Dr. Daszak’s research has been instrumental in identifying and predicting the origins and impact of emerging diseases across the globe. This includes identifying the bat origin of SARS, the drivers of Nipah virus emergence, publishing the first global emerging disease ‘hotspots’ map, discovering SADS coronavirus, designing a strategy to identify the number of unknown viruses in wildlife, launching the Global Virome Project, identifying the first case of a species extinction due to disease, and discovering the disease chytridiomycosis as the cause global amphibian declines. He is one of the founders of the field of Conservation Medicine and has been instrumental in the growth of EcoHealth, One Health, and now Planetary Health.
A fundamental part of the Dr. Daszak’s work on disease ecology is directed by the conviction that disease outbreaks are not just predictable, but preventable. This approach is informed by a perspective on emerging infectious disease research that sees problems of human and animal disease as intimately linked – exacerbated by ecological change. With this in mind, he led the researcher that produced the first ever global emerging disease ‘hotspots’ map to determine where in the world viruses with pandemic potential are most likely to emerge, and developed a strategy to identify just how many of those viruses currently exist.
[left]WHO Expert Peter Daszak Boasts About Manipulation Of Killer SARs Like Virus In Wuhan | India First
|
|
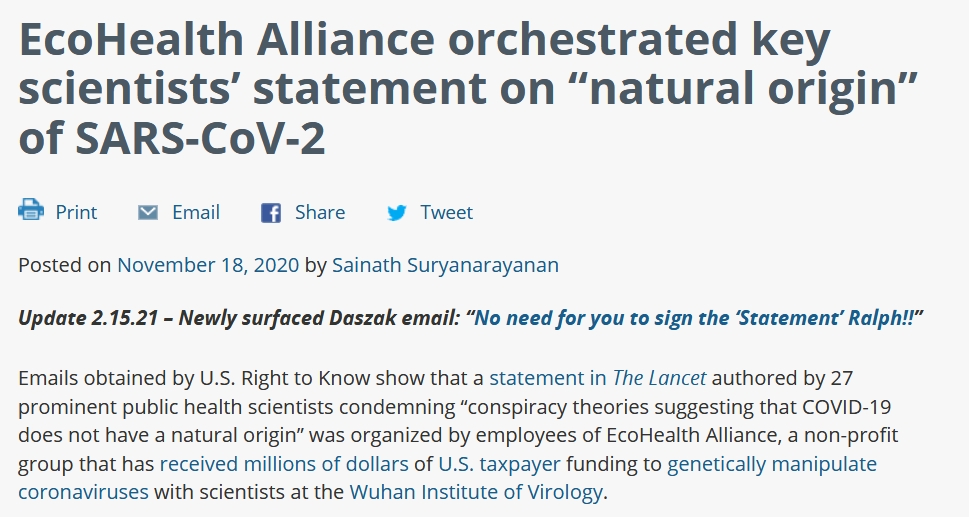
Exposed email shows Peter Daszak, Linfa Wang, and Ralph Baric purposely avoided signing the Lancet statement although Daszak eventually signed likely because of the central role he played in organizing this statement.
|
e emails obtained via public records requests show that EcoHealth Alliance President Peter Daszak drafted the Lancet statement, and that he intended it to “not be identifiable as coming from any one organization or person” but rather to be seen as “simply a letter from leading scientists”. Daszak wrote that he wanted “to avoid the appearance of a political statement”.
The scientists’ letter appeared in The Lancet on February 18, just one week after the World Health Organization announced that the disease caused by the novel coronavirus would be named COVID-19.
The 27 authors “strongly condemn[ed] conspiracy theories suggesting that COVID-19 does not have a natural origin,” and reported that scientists from multiple countries “overwhelmingly conclude that this coronavirus originated in wildlife.” The letter included no scientific references to refute a lab-origin theory of the virus. |

“I’m convinced we’re going to find out fairly soon,” said Peter Daszak, a member of the WHO-led team and a zoologist who specializes in hunting for viral origins in animals. “Within the next few years we’ll have real significant data on where this came from and how it emerged.”
Dr. Daszak was one of three team members who spoke at a webinar organized by Chatham House, an international-affairs think tank in London, about their recent monthlong trip to the Chinese city of Wuhan. The Huanan market there was the site of the first known outbreak of the coronavirus in December 2019.
|
Peter Daszak
Dr. Daszak is a member of the National Academies of Sciences, Engineering, and Medicine's Forum on Microbial Threats, the Academies Advisory Committee to the U.S. Global Change Research Program (USGCRP), the Supervisory Board of the One Health Platform, the One Health Commission Council of Advisors, and the Center of Excellence for Emerging and Zoonotic Animal Diseases (CEEZAD) External Advisory Board. He has served on the Institue of Medicine (IOM) committee on global surveillance for emerging zoonoses, the National Research Council (NRC) committee on the future of veterinary research, the International Standing Advisory Board of the Australian Biosecurity CRC; and has advised the Director for Medical Preparedness Policy on the White House National Security Staff on global health issues. Dr. Daszak won the 2000 CSIRO medal for collaborative research on the discovery of amphibian chytridiomycosis, is the EHA institutional lead for the United States Agency for International Development (USAID) Emerging Pandemic Threats-PREDICT and PREDICT-2, is on the editorial board of Conservation Biology and Transactions of the Royal Society of Tropical Medicine & Hygiene, and is Editor-in-Chief of the journal Ecohealth. He has authored more than 200 scientific papers, and his work has been the focus of extensive media coverage, ranging from popular press articles to television appearances. |
|
|
Four significant insights emerge from all this.
First, although it is called the EcoHealth Alliance, Peter Daszak and his non-profit work closely with the military.
Second, the EcoHealth Alliance attempts to conceal these military connections.
Third, through militaristic language and analogies Daszak and his colleagues promote what is often referred to as, and even then somewhat euphemistically, an ongoing agenda known as “securitization“. In this case it is the securitization of infectious diseases and of global public health. That is, they argue that pandemics constitute a vast and existential threat. They minimize the very real risks associated with their work, and sell it as a billion dollar solution.
The fourth insight is that Daszak himself, as the Godfather of the Global Virome Project, stands to benefit from the likely outlay of public funds.
|
Cold war 2.0 cover story
as China are once again "the bad guys"
who do the 'dirty work' for the agenda...
meanwhile The Bio-genomics global industry
Hand in hand with the respective Military
are growing exponentially
|
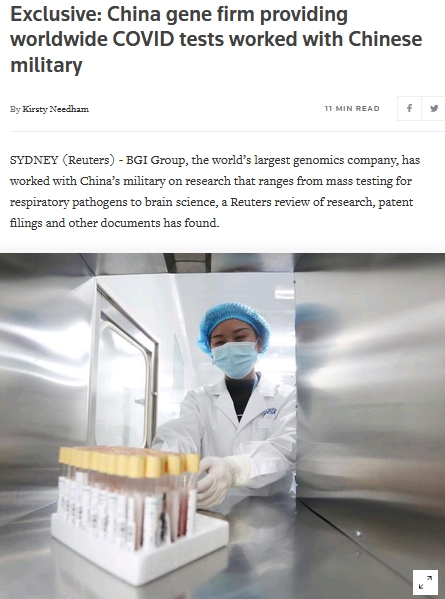
Exclusive: China gene firm providing worldwide COVID tests worked with Chinese military
BGI Group, based in Shenzhen, has grown quickly by selling genetic sequencing services to universities and health systems around the world and amassing a large DNA databank. It created China’s first cloned pig in 2010.
One science paper authored by BGI founders Yang Huanming and Wang Jian along with the PLA’s Key Laboratory of High Altitude Medicine and the Third Military Medical University focused on experiments with the brains of monkeys suffering altitude sickness.
The study, published in January 2020, stated that it was funded as one of the “key projects of military science and technology” by the PLA. A decade ago, the military university’s research sought to identify genes related to altitude sickness so the PLA could screen for susceptible soldiers. The latest research focused on how drugs interacting with genes could potentially protect a person from brain injury. |

|
BGI Group, described in one 2015 study as “Goliath” in the fast-growing field of genomics research, is using an opening created by the pandemic to expand its footprint globally. In the past six months, it says it has sold 35 million rapid COVID-19 testing kits to 180 countries and built 58 labs in 18 countries. Some of the equipment has been donated by BGI’s philanthropic arm, promoted by China’s embassies in an extension of China’s virus diplomacy.
But as well as test kits, the company is distributing gene-sequencing technology that U.S. security officials say could threaten national security. This is a sensitive area globally. Sequencers are used to analyse genetic material, and can unlock powerful personal information.
In science journals and online, BGI is calling on international health researchers to send in virus data generated on its equipment, as well as patient samples that have tested positive for COVID-19, to be shared publicly via China’s government-funded National GeneBank.
As BGI’s foothold in the gene-sequencing industry grows, a senior U.S. administration official told Reuters on condition of anonymity, so does the risk China could harvest genetic information from populations around the world.
Underpinning BGI’s global expansion are the Shenzhen-based company’s links to the Chinese government, which include its role as operator of China’s national genetic database and its research in government-affiliated key laboratories. BGI, which says in stock market filings it aims to help the ruling Communist Party achieve its goal to “seize the commanding heights of international biotechnology competition,” is coming under increasing scrutiny in an escalating Cold War between Washington and Beijing, Reuters found.
|
|
Reuters found no evidence that BGI is violating patient privacy protections where these apply. Responding to questions from the news agency, BGI said it is not owned by the Chinese government.
“Under the current political climate, the fear raised about the use of BGI’s technology is unfounded and misleading,” BGI said in a statement to Reuters. “BGI’s mission is, and has always been, using genomics to benefit people’s health and wellbeing.”
China’s foreign ministry said in a statement the country has been open, transparent and responsible in “sharing information and experience with the international community, providing supplies to relevant countries” including COVID-19 test kits and protective equipment, and helping countries improve epidemic control.
The extent of BGI’s endeavours to dominate an industry with geostrategic value, as well as of its efforts to gather genetic data from around the world, was pieced together by Reuters from public documents and dozens of interviews with scientists, researchers and health officials.
Some U.S. officials warn of a dual risk to national security from BGI: Sensitive genetic information about U.S. citizens may fall into foreign hands, and American companies stand to lose their innovative edge in the field of genomics to Chinese firms.
|
|
|
Earlier this year, the U.S. National Counterintelligence and Security Center (NCSC) published practical tips for health services to avoid “potential threats posed by foreign powers” in connection with COVID-19 tests. Other officials draw parallels between BGI and Huawei Technologies Co., the Chinese telecommunications titan whose 5G technology the United States says could be used to capture personal data that Beijing could exploit. Huawei has said it would refuse to cooperate with spying.
Sharing data is essential for medical research. But in the case of genetic data, officials and scientists say the risks are that it could be weaponized.
Individuals can be identified by a portion of their DNA, and some researchers have found genetic links with behaviours such as depressive disorder. A hostile actor could use such data to target individuals for surveillance, extortion or manipulation, according to a comprehensive report prepared for the U.S. Office of the Director of National Intelligence by science and medical experts in January, which added that such associations are not yet well understood.
|
|
Knowledge of the genetic makeup of national decision-makers or the military, and their propensity to act in certain ways, could be used by adversarial intelligence agencies as a mechanism of influence, said the report, “Safeguarding the Bioeconomy,” from the National Academies of Sciences, Engineering and Medicine. Genetic data could reveal a U.S. vulnerability to specific diseases, it added.
As companies race to develop and patent biological drugs for the global market, the ethnic diversity of the U.S. population makes U.S. genomic data more valuable than data from countries with homogeneous populations, the report said. That’s because the more varied the data, the bigger the advantage in identifying genetic disease. The report raised the possibility BGI could amass DNA sequence information from U.S. genetic samples that would give it an “asymmetrical” advantage over U.S. firms.
Genetic information, including family medical history, “is of enormous value and can be exploited by foreign regimes for a range of security and economic purposes,” Bill Evanina, director of the NCSC, told Reuters in response to questions about Chinese genomic companies.
BGI and Huawei have said they work together. In a video that is no longer available on Huawei’s site, a BGI executive said it processes “staggering volumes of data” from its gene sequencers, stored on Huawei’s high-powered systems. In response to questions from Reuters about whether this information could be shared with China’s government, Huawei said only users of its technology can define who to share data with. “Huawei’s Cloud technology and cloud computing services are secure and compliant with international security standards,” it said, adding it complies with all laws.
BGI said it does not have access to patient data from its diagnostic tests.
The company said it is conducting scientific research on the genomes, or genetic blueprints, of the virus and patients with COVID-19. But it said this research is separate from the tests it provides to other nations to diagnose COVID-19.
Asked about China’s genomics ambitions, a U.S. State Department spokesman said: “We believe countries need to be able to trust that vendors will not threaten national security, privacy or intellectual property. Trust cannot exist where a company is subject to an authoritarian government, like the People’s Republic of China, that lacks prohibitions on the misuse of data.”
FROM “WHO1” TO FIRE EYE
BGI was involved in China’s response to the coronavirus from the start. Its scientists were among teams that sequenced the virus genome and shared that information in January.
On Dec. 26, BGI collected and tested a throat swab from a 44-year-old man who was a patient in the military hospital in Wuhan, according to a record of the sequence that was shared with other researchers on a global database. The World Health Organisation (WHO) learned of cases of pneumonia of an unknown cause in Wuhan on Dec. 31; the blueprint of the patient’s virus was named WHO1.
The week after that first test, BGI took swabs from another three patients at the hospital who had been to the local seafood market, according to a paper published by Chinese scientists in The Lancet on Jan. 29. BGI sequenced these samples.
By month’s end, the company had designed an automated laboratory for the Wuhan government to massively increase testing. BGI called the design “Fire Eye,” after the ability of China’s fabled Monkey King to see disguised threats.
The labs were replicated in China, and the Mammoth Foundation – a charity established a few months earlier by BGI – started donating tests and laboratories worldwide. By mid-year, BGI’s COVID-19 lab equipment was installed in at least 10 countries thanks to donations by the charity, company statements and local news reports show.
China’s government helped coordinate some of BGI’s deals. The Solomon Islands said it had received a $300,000 cheque from the Chinese embassy, which advised the island nation to buy tests and lab equipment from BGI. China’s foreign ministry said China has done its best to ensure safety and reliability of medical supplies.
Besides donations, BGI has reported lab deals worth hundreds of millions of dollars. As technicians in white protective suits build Fire Eye labs in countries from Australia to Saudi Arabia, BGI Genomics, a listed subsidiary of the group, said last month demand would help boost profits by 700% for the first half of the year to more than $218 million.
POWERFUL INFORMATION
There are two main aspects to BGI’s COVID-19 programme.
First, diagnostic test kits, which come with high-speed processing robots to handle large volumes, work by detecting the genetic material of the virus in a patient’s sample to tell if a person has been infected. BGI says these tests do not give access to patient data.
The second part, which the company offers as an add-on in its marketing materials, is gene-sequencing equipment.
In the pandemic, researchers around the globe are using sequencers to track mutations in the virus, see which mutation is spreading, and choose strains or samples to work with for vaccine development.
Underpinning demand for DNA sequencers is also their role driving a lucrative medical field known as precision or personal medicine.
Rather than seek one-drug-suits-all treatments, precision medicine focuses on how different people’s genes interact with their environment to help predict their risk of disease, or their response to medications.
In July, BGI Genomics, BGI’s listed subsidiary on the Shenzhen stock exchange filed for a $293 million capital hike, telling investors in the filing their support would help it collect as much patient data as possible, “on the human body, genome, people’s living habits and environment, so we can understand more, and diagnose in a more precise way.”
The company also says it plans to promote the Fire Eye labs it rolls out for COVID-19 for precision medicine after the pandemic.
FROM CUSTOMER TO RIVAL
BGI was set up by four scientists in 1999 as a non-profit research body called the Beijing Genomics Institute, to enable China to join a global project to map the human genome. Since 2016, its headquarters have housed and operated the government-funded China National GeneBank, a biorepository of 20 million plant, animal and human genetic samples.
In 2010, BGI received a $1.5 billion loan from the state-run China Development Bank, some of which it used to buy 128 sequencing machines from an American firm, San Diego-based Illumina Inc.
Two years after that, Beijing said in a State Council plan for the bio-industry that it wanted China to develop gene sequencing technology. In 2013, BGI succeeded in buying Illumina’s largest competitor, California-based Complete Genomics, for $118 million. That is now the U.S. research arm of the Chinese group. BGI Group launched its own sequencing equipment in 2015; the group floated BGI Genomics in 2017.
This year, BGI Genomics told investors that it had cost $95 million to sequence a whole human genome in 2001. By 2014, Illumina had announced it reduced the cost to below $1,000. Now BGI could do it for $600.
In May, MGI Tech, the BGI subsidiary that makes DNA sequencers, raised $1 billion in venture capital.
But after BGI indicated it would launch its sequencers in the United States, it ran into a challenge - an accusation of intellectual property violations from Illumina. In June, a U.S. court issued a preliminary injunction banning the sale, distribution or promotion of BGI’s materials and equipment, pending a trial to decide if the technology was copied from Illumina.
Seeking the injunction, Illumina’s lawyers told the court: “BGI plans extreme price cutting and ambitious sales directly against Illumina.”
BGI declined to comment on the case. In court papers, BGI denied infringing Illumina’s patents and asked that parts of the injunction be put on hold while it appealed the ruling. Illumina told Reuters COVID-19 will boost sequencer demand.
“MORE VALUABLE THAN GOLD”
With a price tag of between $20,000 for a portable model and $1 million for a powerful machine, gene sequencers are an important part of a country’s pandemic armoury.
Even before the new coronavirus, in October 2019, Ethiopia’s government said it would establish a genomics lab with equipment donated by BGI. Months later, Illumina donated sequencers to 10 African nations to help monitor the virus, the U.S. company said.
At least five countries worldwide have received BGI’s sequencers with the Fire Eye labs, according to statements the countries or BGI have released. In many cases BGI does not own or operate the Fire Eye laboratories, but simply provides the equipment, the company told Reuters.
For BGI, sequencers offer more than money. It has said they will also help it study the virus in large populations.
One recipient of BGI sequencing equipment is Serbia, the Balkan country where Beijing has invested heavily as part of its One Belt, One Road initiative to open trade links for Chinese companies. Two labs have opened there. Both were donated by Chinese companies, Beijing and Belgrade said.
After the first lab opened, coordinator Jelena Begovic told Reuters in May that DNA sequencers help researchers by linking genetic information on the virus with genetic information on the patient. In future, she said, the labs would underpin cooperation with BGI.
“Information is nowadays sometimes more valuable than gold,” she said. “In that sense, this is also a source of information for them regarding this region.”
Prime Minister Ana Brnabic told an opening ceremony that after the pandemic, “We will have the most modern lab, which will enable us to start talking with BGI on how to build the most advanced institute for precision medicine and genetics in this region.”
Sweden, too, has received sequencing equipment from BGI. The Karolinska Institute, a medical university in Stockholm, hopes to use it to identify a human genotype that is more susceptible to the disease, microbiology professor Lars Engstrand said in a presentation on BGI’s website.
Asked by Reuters about the risk Swedish genomic data may be collected by the Chinese government, Engstrand said this was “a very relevant question” and the institute’s IT security department had scrutinised its collaboration with BGI.
“There will be no sequence information sent to any other servers or computers outside our institute,” said Engstrand, who heads the institute’s Center for Translational Microbiome Research, in an email. “No cloud solution will be used for these sensitive data.”
He was unsure if the institute would go ahead with sequencing human genomes, he added.
“CONSIDERABLE SUPPORT”
Researchers globally are sharing virus data, but BGI has also set up its own sharing platform, the “Global Initiative on Open-source Genomics” for the new coronavirus.
On a website together with the China National GeneBank, giogs.genomics.cn, it invites international scientists to send in virus information including patient age, gender and location, collected in accordance with local regulations.
“You will be asked to share virus genome data to the public via (the National GeneBank) in the first instance,” the site says.
In exchange, the site offers sequencing services and “considerable support” for the cost of kits and reagents.
BGI told Reuters it had received no patient samples under this new programme - samples have been sequenced in local facilities.
It said its goal is to “develop more high-quality genome data of (the) virus with BGI’s sequencing solution.” It said it wants to facilitate the rapid and open sharing of genome data to support research on the virus.
Besides the National GeneBank, BGI’s headquarters also house at least four government-designated “Key Laboratories” for genomic research, which are also government-funded. BGI said this funding is used for research, not operations.
One of the labs supported a study by a dozen BGI researchers who sequenced the genomes of more than 300 COVID-19 patients in a Shenzhen hospital, according to a paper they shared on MedRvix, a website for pre-published scientific papers.
“We and the others are continuing to recruit patients and data in China and around the world to understand the host genetic background underlying the varying clinical outcome of the patients,” the researchers wrote.
As rapid COVID-19 tests are adopted globally, the researchers added, it will be important to study patients who don’t show symptoms. The study’s lead author didn’t respond to questions from Reuters.
SECURITY APPARATUS
BGI’s pandemic push comes as tensions between China and the United States are mounting, including over China’s genetic programme.
Two BGI subsidiaries were blacklisted by the U.S. Commerce Department last month for China’s alleged human rights violations. Washington alleged BGI is involved in conducting genetic analysis of Uighur Muslims in Xinjiang in western China, where U.N. experts and activists say Muslims were held in detention centres.
BGI said in a statement it “does not condone and would never be involved in any human-rights abuses.” Chinese officials say the camps are educational and vocational institutions and deny they violate the human rights of the detainees.
China’s security apparatus is a BGI customer. Another BGI subsidiary, Forensic Genomics International, says on its website it works with China’s Public Security Bureau. It had multiple contracts with the police to collect male DNA samples, as well as samples from some newborn babies, a survey this year by the Australian Strategic Policy Institute found.
BGI said the forensics subsidiary complied with scientific ethics and the law. The foreign ministry declined to comment.
|
|
Dr. Charles Lieber
According to court documents, since 2008, Dr. Lieber who has served as the Principal Investigator of the Lieber Research Group at Harvard University, which specialized in the area of nanoscience, has received more than$15,000,000 in grant funding from the National Institutes of Health (NIH) and Department of Defense (DOD).
These grants require the disclosure of significant foreign financial conflicts of interest, including financial support fromforeign governments or foreign entities. Unbeknownst to Harvard University beginning in 2011, Lieber became a“Strategic Scientist” at Wuhan University of Technology (WUT) in China and was a contractual participant in China’s Thousand Talents Plan from in or about 2012 to 2017.
China’s Thousand Talents Plan is one of the most prominent Chinese Talent recruit plans that are designed to attract, recruit, and cultivate high-level scientific talent infurtherance of China’s scientific development, economic prosperity and national security.
These talent programsseek to lure Chinese overseas talent and foreign experts to bring their knowledge and experience to China andreward individuals for stealing proprietary information.
Under the terms of Lieber’s three-year Thousand Talentscontract, WUT paid Lieber $50,000 USD per month, living expenses of up to 1,000,000 Chinese Yuan(approximately $158,000 USD at the time) and awarded him more than $1.5 million to establish a research lab at WUT.
In return, Lieber was obligated to work for WUT “not less than nine months a year” by “declaring internationalcooperation projects, cultivating young teachers and Ph.D. students, organizing international conference[s],applying for patents and publishing articles in the name of” WUT.The complaint alleges that in 2018 and 2019, Lieber lied about his involvement in the Thousand Talents Plan andaffiliation with WUT
Charles Lieber arrested Tuesday, January 28, 2020 Dept of Justice
Kinked p-n Junction Nanowire Probes for High Spatial ResolutionSensing and Intracellular Recording
|
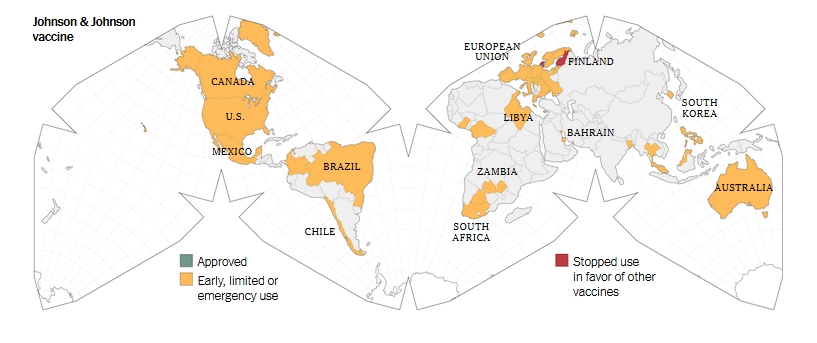
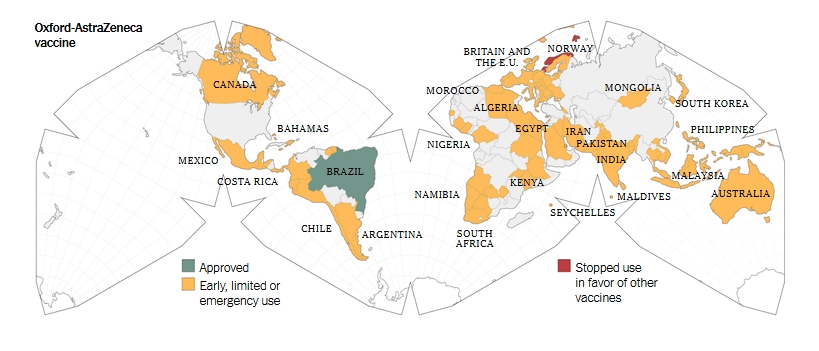
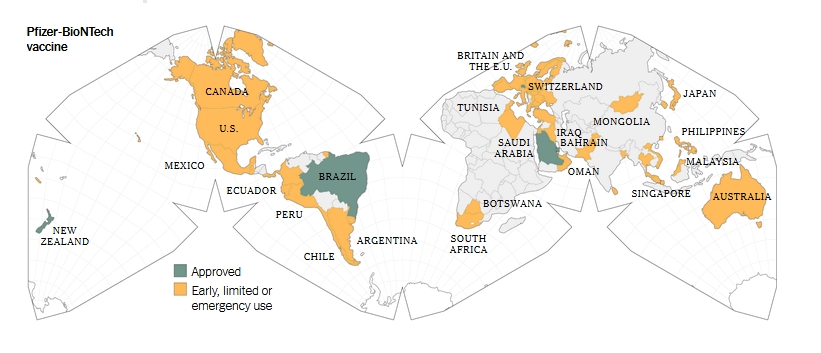
Informed consent disclosure to vaccine trial subjects of risk of COVID-19 vaccines worsening clinical disease
Results of the study: COVID-19 vaccines designed to elicit neutralising antibodies may sensitise vaccine recipients to more severe disease than if they were not vaccinated. Vaccines for SARS, MERS and RSV have never been approved, and the data generated in the development and testing of these vaccines suggest a serious mechanistic concern: that vaccines designed empirically using the traditional approach (consisting of the unmodified or minimally modified coronavirus viral spike to elicit neutralising antibodies), be they composed of protein, viral vector, DNA or RNA and irrespective of delivery method, may worsen COVID-19 disease via antibody-dependent enhancement (ADE). This risk is sufficiently obscured in clinical trial protocols and consent forms for ongoing COVID-19 vaccine trials that adequate patient comprehension of this risk is unlikely to occur, obviating truly informed consent by subjects in these trials.
|
|
|
|
|
Will covid-19 vaccines save lives?
BMJ 2020; 371 doi: https://doi.org/10.1136/bmj.m4037 (Published 21 October 2020) Cite this as:
British medical Journal - BMJ 2020;371:m4037
Linked Editorial
- Peter Doshi, associate editor - pdoshi@bmj.com
Covid-19 vaccine trial protocols released
The world has bet the farm on vaccines as the solution to the pandemic, but the trials are not focused on answering the questions many might assume they are. Peter Doshi reports
As phase III trials of covid-19 vaccines reach their target enrolments, officials have been trying to project calm. The US coronavirus czar Anthony Fauci and the Food and Drug Administration leadership have offered public assurances that established procedures will be followed.1234 Only a "safe and effective" vaccine will be approved, they say, and nine vaccine manufacturers issued a rare joint statement pledging not to prematurely seek regulatory review.5
But what will it mean exactly when a vaccine is declared "effective"? To the public this seems fairly obvious. "The primary goal of a covid-19 vaccine is to keep people from getting very sick and dying," a National Public Radio broadcast said bluntly.6
Peter Hotez, dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, said, "Ideally, you want an antiviral vaccine to do two things . . . first, reduce the likelihood you will get severely ill and go to the hospital, and two, prevent infection and therefore interrupt disease transmission."7
Yet the current phase III trials are not actually set up to prove either (table 1). None of the trials currently under way are designed to detect a reduction in any serious outcome such as hospital admissions, use of intensive care, or deaths. Nor are the vaccines being studied to determine whether they can interrupt transmission of the virus.
Table 1
Characteristics of ongoing phase III covid-19 vaccine trials
Evaluating mild, not severe, disease
In a September interview Medscape editor in chief Eric Topol pondered what counts as a recorded "event" in the vaccine trials. "We’re not talking about just a PCR [polymerase chain reaction test]-positive mild infection. It has to be moderate to severe illness to qualify as an event, correct?" he asked.8
"That’s right," concurred his guest, Paul Offit, a vaccinologist who sits on the FDA advisory committee that may ultimately recommend the vaccines for licence or emergency use authorisation.
But that’s not right. In all the ongoing phase III trials for which details have been released, laboratory confirmed infections even with only mild symptoms qualify as meeting the primary endpoint definition.9101112 In Pfizer and Moderna’s trials, for example, people with only a cough and positive laboratory test would bring those trials one event closer to their completion. (If AstraZeneca’s ongoing UK trial is designed similarly to its "paused" US trial for which the company has released details, a cough and fever with positive PCR test would suffice.)
Part of the reason may be numbers. Severe illness requiring hospital admission, which happens in only a small fraction of symptomatic covid-19 cases, would be unlikely to occur in significant numbers in trials. Data published by the US Centers for Disease Control and Prevention in late April reported a symptomatic case hospitalisation ratio of 3.4% overall, varying from 1.7% in 0-49 year olds and 4.5% in 50-64 year olds to 7.4% in those 65 and over.13 Because most people with symptomatic covid-19 experience only mild symptoms,14 even trials involving 30?000 or more patients would turn up relatively few cases of severe disease.
In the trials, final efficacy analyses are planned after just 150 to 160 "events,"—that is, a positive indication of symptomatic covid-19, regardless of severity of the illness.
Yet until vaccine manufacturers began to release their study protocols in mid-September, trial registries and other publicly released information did little to dispel the notion that it was severe covid-19 that the trials were assessing. Moderna, for example, called hospital admissions a "key secondary endpoint" in statements to the media.15 And a press release from the US National Institutes of Health reinforced this impression, stating that Moderna’s trial "aims to study whether the vaccine can prevent severe covid-19" and "seeks to answer if the vaccine can prevent death caused by covid-19."16
But Tal Zaks, chief medical officer at Moderna, told The BMJ that the company’s trial lacks adequate statistical power to assess those outcomes. "The trial is precluded from judging [hospital admissions], based on what is a reasonable size and duration to serve the public good here," he said.
Hospital admissions and deaths from covid-19 are simply too uncommon in the population being studied for an effective vaccine to demonstrate statistically significant differences in a trial of 30?000 people. The same is true of its ability to save lives or prevent transmission: the trials are not designed to find out.
Zaks said, "Would I like to know that this prevents mortality? Sure, because I believe it does. I just don’t think it’s feasible within the timeframe [of the trial]—too many would die waiting for the results before we ever knew that."
Stopping transmission
What about Hotez’s second criterion, interrupting virus transmission, which some experts have argued17 should be the most important test in phase III studies?
"Our trial will not demonstrate prevention of transmission," Zaks said, "because in order to do that you have to swab people twice a week for very long periods, and that becomes operationally untenable."
He repeatedly emphasised these "operational realities" of running a vaccine trial. "Every trial design, especially phase III, is always a balancing act between different needs," he said. "If you wanted to have an answer on an endpoint that happens at a frequency of one 10th or one fifth the frequency of the primary endpoint, you would need a trial that is either 5 or 10 times larger or you’d need a trial that is 5 or 10 times longer to collect those events. Neither of these, I think, are acceptable in the current public need for knowing expeditiously that a vaccine works."
Zaks added, "A 30?000 [participant] trial is already a fairly large trial. If you’re asking for a 300 000 trial then you need to talk to the people who are paying for it, because now you’re talking about not a $500m to $1bn trial, you’re talking about something 10 times the size. And I think the public purse and operational capabilities and capacities we have are rightly spent not betting the farm on one vaccine but, as Operation Warp Speed [the US government’s covid-19 vaccine plan] is trying to do, making sure that we’re funding several vaccines in parallel."
Debating endpoints
Still, it’s fair to say that most of the general public assumes that the whole point of the current trials, besides testing safety (box 1), is to see whether the vaccine can prevent bad outcomes. "How do you reconcile that?" The BMJ asked Zaks.
Box 1
Safety and side effects
History shows many examples of serious adverse events from vaccines brought to market in periods of enormous pressure and expectation. There were contaminated polio vaccines in 1955, cases of Guillain-Barré syndrome in recipients of flu vaccines in 1976, and narcolepsy linked to one brand of influenza vaccine in 2009.1819
"Finding severe rare adverse events will require the study of tens of thousands of patients, but this requirement will not be met by early adoption of a product that has not completed its full trial evaluation," Harvard drug policy researchers Jerry Avorn and Aaron Kesselheim recently wrote in JAMA.20
Covid-19 vaccine trials are currently designed to tabulate final efficacy results once 150 to 160 trial participants develop symptomatic covid-19—and most trials have specified at least one interim analysis allowing for the trials to end with even fewer data accrued.
Medscape’s Eric Topol has been a vocal critic of the trials’ many interim analyses. "These numbers seem totally out of line with what would be considered stopping rules," he says. "I mean, you’re talking about giving a vaccine with any of these programmes to tens of millions of people. And you’re going to base that on 100 events?"8
Great uncertainty remains over how long a randomised trial of a vaccine will be allowed to proceed. If efficacy is declared, one possibility is that the thousands of volunteers who received a saline placebo would be offered the active vaccine, in effect ending the period of randomised follow-up. Such a move would have far reaching implications for our understanding of vaccines’ benefits and harms, rendering uncertain our knowledge of whether the vaccines can reduce the risk of serious covid-19 disease and precluding any further ability to compare adverse events in the experimental versus the placebo arm.
"It’ll be a decision we’ll have to take at that time. We have not committed one way or another," Moderna’s Tal Zaks told The BMJ. "It will be a decision where FDA and NIH will also weigh in. And it will be probably a very difficult decision, because you will be weighing the benefit to the public in continuing to understand the longer term safety by keeping people on placebo and the expectation of the people who have received placebo to be crossed over now that it has been proved effective."
"Very simply," he replied. "Number one, we have a bad outcome as our endpoint. It’s covid-19 disease." Moderna, like Pfizer and Janssen, has designed its study to detect a relative risk reduction of at least 30% in participants developing laboratory confirmed covid-19, consistent with FDA and international guidance.2122
Number two, Zaks pointed to influenza vaccines, saying they protect against severe disease better than mild disease. To Moderna, it’s the same for covid-19: if its vaccine is shown to reduce symptomatic covid-19, it will be confident it also protects against serious outcomes.
But the truth is that the science remains far from clear cut, even for influenza vaccines that have been used for decades. Although randomised trials have shown an effect in reducing the risk of symptomatic influenza, such trials have never been conducted in elderly people living in the community to see whether they save lives.
Only two placebo controlled trials in this population have ever been conducted, and neither was designed to detect any difference in hospital admissions or deaths.23 Moreover, dramatic increases in use of influenza vaccines has not been associated with a decline in mortality (box 2).26
Box 2
Not enrolling enough elderly people or minorities
A vaccine that has been proved to reduce the risk of symptomatic disease by a certain proportion should, you might think, reduce serious outcomes such as hospital admissions and deaths in equal proportion.
Peter Marks, an FDA official with responsibility over vaccine approvals, recently stated as much about influenza vaccination, which "only prevents flu in about half the people who get it. And yet that’s very important because that means that it leads to half as many deaths related to influenza each year."24
But when vaccines are not equally effective in all populations the theory breaks down.
If frail elderly people, who are understood to die in disproportionate numbers from both influenza25 and covid-19, are not enrolled into vaccine trials in sufficient numbers to determine whether case numbers are reduced in this group, there can be little basis for assuming any benefit in terms of hospital admissions or mortality. Whatever reduction in cases is seen in the overall study population (most of which may be among healthy adults), this benefit may not apply to the frail elderly subpopulation, and few lives may be saved.
This is hard to evaluate in the current trials because there are large gaps in the types of people being enrolled in the phase III trials (table 1). Despite recruiting tens of thousands, only two trials are enrolling children less than 18 years old. All exclude immunocompromised people and pregnant or breastfeeding women, and though the trials are enrolling elderly people, few or perhaps none of the studies would seem to be designed to conclusively answer whether there is a benefit in this population, despite their obvious vulnerability to covid-19.
"Adults over 65 will be an important subgroup that we will be looking at," Moderna’s Zaks told The BMJ. "That said . . . any given study is powered for its primary endpoint—in our case covid-19 disease irrespective of age."
Al Sommer, dean emeritus of the Johns Hopkins School of Public Health, told The BMJ, "If they have not powered for evidence of benefit in the elderly, I would find that a significant, unfortunate shortcoming." He emphasised the need for "innovative follow-up studies that will enable us to better determine the direct level of protection immunisation has on the young and, separately, the elderly, in addition to those at the highest risk of severe disease and hospitalisation."
One view is that trial data should be there for all target populations. "If we don’t have adequate data in the greater than 65 year old group, then the greater than 65 year old person shouldn’t get this vaccine, which would be a shame because they’re the ones who are most likely to die from this infection," said vaccinologist Paul Offit.8 "We have to generate those data," he said. "I can’t see how anybody—the Data and Safety Monitoring Board or the FDA Vaccine Advisory Committee, or FDA decision-makers—would ever allow a vaccine to be recommended for that group without having adequate data."
"I feel the same way about minorities," Offit added. "You can’t convince minority populations to get this vaccine unless they are represented in these trials. Otherwise, they’re going to feel like they’re guinea pigs, and understandably so."
|
|
| "The E484K amino acid change, a receptor-binding-domain (RBD) mutation, was reported to be "associated with escape from neutralising antibodies" which could adversely affect the efficacy of spike protein-dependent COVID vaccines.[25][26] The E484K spike mutation was linked to a case of reinfection with the Beta variant of SARS-CoV-2 in Brazil, believed by researchers to be the first such case of reinfection involving this mutation" wikipedia |
|
To date severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)
has infected nearly 100 million individuals resulting in over two million deaths. Many vaccines are being deployed to prevent coronavirus disease-2019 (COVID-19) including two novel mRNA-based vaccines1,2.
These vaccines elicit neutralizing antibodies and appear to be safe and effective, but the precise nature of the elicited antibodies is not known3–5. Here we report on the antibody and memory B cell responses in a cohort of 20 volunteers who received either the Moderna (mRNA-1273) or Pfizer-BioNTech (BNT162b2) vaccines.
Consistent with prior reports, 8 weeks after the second vaccine injection volunteers showed high levels of IgM, and IgG anti-SARS-CoV-2 spike protein (S), receptor binding domain (RBD) binding titers3,5.
Moreover, the plasma neutralizing activity, and the relative numbers of RBD-specific memory B cells were equivalent to individuals who recovered from natural infection6,7.
However, activity against SARS-CoV-2 variants encoding E484K or N501Y or the K417N:E484K:N501Y combination was reduced by a small but significant margin.
Consistent with these findings, vaccine-elicited monoclonal antibodies (mAbs) potently neutralize SARS-CoV-2, targeting a number of different RBD epitopes epitopes in common with mAbs isolated from infected donors.
Structural analyses of mAbs complexed with S trimer suggest that vaccine- and virus-encoded S adopts similar conformations to induce equivalent anti-RBD antibodies.
However, neutralization by 14 of the 17 most potent mAbs tested was reduced or abolished by either K417N, or E484K, or N501Y mutations.
Notably, the same mutations were selected when recombinant vesicular stomatitis virus (rVSV)/SARS-CoV-2 S was cultured in the presence of the vaccine elicited mAbs.
Taken together the results suggest that the monoclonal antibodies in clinical use should be tested against newly arising variants, and that mRNA vaccines may need to be updated periodically to avoid potential loss of clinical efficacy.
|
Coincidences between mass vaccination rollout and new variants emerging
The first three significant new variants emerged from Brazil, South Africa and the UK which were all sites of vaccine trials. There have since been further variants which have appeared after vaccination roll out in several other countries. Some experts have speculated on the coincidence of such events and this phenomenon is currently being studied. In one study recently posted as a preprint and not yet formally reviewed, Theodora Hatziioannou, a virologist at Rockefeller University in New York, and her colleagues created a ‘pseudo-coronavirus’ carrying a non-variant version of the spike protein. This was grown in the presence of individual antibodies extracted from the blood of people who had received one of the two FDA-authorized COVID-19 vaccines, one from Pfizer/BioNTech and one from Moderna.
Some antibodies spurred the pseudo-SARS-CoV-2 to acquire various mutations.
They tried the experiment again with no antibodies present and none of the three mutations — the ones in the triple-variant threat — evolved the same evasive manoeuvres.
“This data shows that these mutations accumulating in the spike protein are antibody escape mutations,” says Hatziioannou. “As soon as you add a specific antibody, you see specific mutations.”
Hatziioannou and others think there are also clues to be found in the genomes of viruses that took up long-term residence in the bodies of immunocompromised COVID-19 patients. The prevailing theory was that escape mutations could have emerged in people with chronic infections, who might be receiving monoclonal antibody treatments or convalescent plasma, and therefore supercharging the selective pressures the virus has to contend with.
By Dr Gerry Quinn
Post-doctoral Researcher in Microbiology and Immunology
|
|
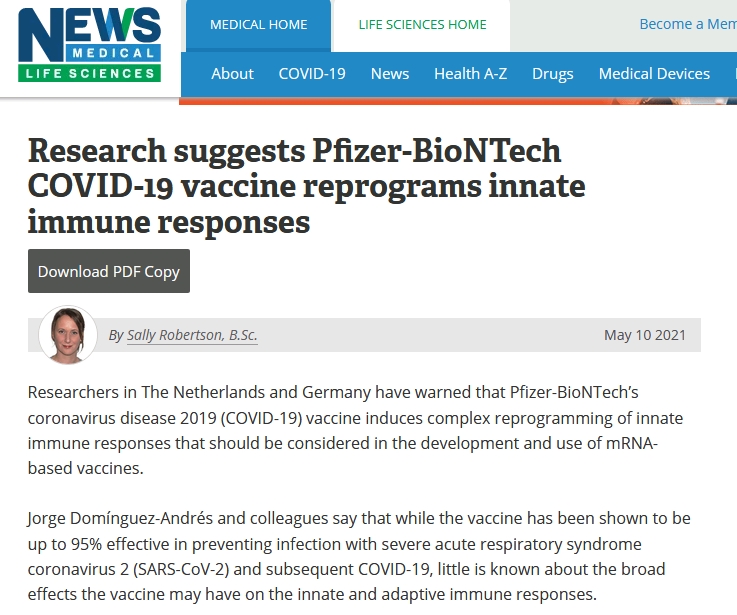
Summary
The mRNA-based BNT162b2 vaccine from Pfizer/BioNTech was the first registered COVID-19 vaccine and has been shown to be up to 95% effective in preventing SARS-CoV-2 infections. Little is known about the broad effects of the new class of mRNA vaccines, especially whether they have combined effects on innate and adaptive immune responses. Here we confirmed that BNT162b2 vaccination of healthy individuals induced effective humoral and cellular immunity against several SARS-CoV-2 variants. Interestingly, however, the BNT162b2 vaccine also modulated the production of inflammatory cytokines by innate immune cells upon stimulation with both specific (SARS-CoV-2) and non-specific (viral, fungal and bacterial) stimuli. The response of innate immune cells to TLR4 and TLR7/8 ligands was lower after BNT162b2 vaccination, while fungi-induced cytokine responses were stronger. In conclusion, the mRNA BNT162b2 vaccine induces complex functional reprogramming of innate immune responses, which should be considered in the development and use of this new class of vaccines.
|
The Concern for the Secretive US Bio-Geopolitics
Oct 10, 2019 - Dan Steinbock
Founder, Difference Group
excerpt -
"...the neoconservative Project for New American Century (PNAC), the ideological force behind the subsequent Bush administration's foreign policy, declared in its manifesto, Rebuilding America's Defenses (2000), that 'advanced forms of biological warfare that can 'target' specific genotypes may transform biological warfare from the realm of terror to a politically useful tool."
Previously, such efforts at biological 'ethnic bombs"had occurred mainly in apartheid-era South Africa and Rhodesia; the PNAC builds on the Israeli 'ethno-bomb'idea to target specific genetic traits among target populations.
By May 2007, Russia banned all exports of human bio samples due to concern for "genetic bio-weapons" targeting the Russian population. Reportedly, some of these institutions, including Harvard Public Health and USAID, have collected biological material in China as well. In October 2018, Russian Defense Ministry claimed the spread of viral diseases from Georgia, including African swine fever since 2007, could be connected to a US lab network in the area, where more than 70 Georgians had died in odd conditions.
The lab network, a branch of the Nunn-Lugar bio-initiative, belongs to the multimillion-dollar Cooperative Biological Engagement Program (CBEP) funded by Pentagon's Cooperative Threat Reduction Agency (DTRA). The CBEP labs are located in 25 countries, including in Eastern Europe (e.g., Georgia and Ukraine), the Middle East, Africa, and Southeast Asia. In several locations, there have been reported outbreaks of tropical diseases, which are not endemic to the area.
Despite high-level Russian calls for a 'comprehensive evaluation"and 'joint inspections,"pleas for multilateral cooperation have been ignored. In its 2020 multimillion-dollar budget, the DTRA characterizes the "bio-security" program in Asia as 'the partner of choice in a region competing against Chinese influence.'
|

"...advanced forms of biological warfare that can "target" specific genotypes may transform biological warfare from the realm of terror to a politically useful tool."
Rebuilding Americas defenses - The Project for the New American Century - 2000
|
|
quote: "the vaccine makes the dangerous part of the virus in the body" - PETER MCCULLOUGH MD
|
what dangerous part? if covid is only dangerous to vulnerable people ... IE 0.02 of the population...so the question needs to be asked - what spike protiein is this mrna gene therapy producing - and is it actually a bioweapon?
"As this, in a sense, bioterrorism phase one was rolled out, it was really all about keeping the population in fear and in isolation and preparing them to accept the vaccine, which appears to be phase two of a bioterrorism operation,"
DR, PETER MCCULLOUGH INTERVIEWED BY REINER FUELLMICH, JUNE 11, 2021 and stating that he believes the vaccine is a BIOWEAPON [video]
Dr Peter McCullough
MD testifies to Texas Senate HHS
|
SELF-TRANSMITTING? or transmitting a variant?
The Outrider Journal
FIVE MAJOR COVID-19 VACCINES MAY BE SELF-TRANSMITTING
brion frantz May 3, 2021 #COVAX / COVAX shedding / Vaccines
We know that for over two years, it has been technically possible for pharmaceutical companies to engineer and manufacture viral vaccines that may transmit like any virus.
No evidence has yet been revealed that any of the COVID-19 vaccines are actually designed to act as a self-spreading vaccine, but there are vaccines that do so that are already in use, and more planned. In an Opinion article titled “Transmissible Viral Vaccines” from the January 2018 of Trends in Microbiology magazine, biologists James J. Bull, Mark W. Smithson and Scott L. Nuismer explain that many viral vaccines are already in use:
Transmissible Viral Vaccines
Two types of live viral vaccine are amenable to transmission: attenuated and recombinant vector vaccines.
The epidemiological consequences of vaccine transmission vary with vaccine design and are often case-specific.
Recombinant vector vaccines offer the greatest and least appreciated potential for transmission.
Vaccine evolution is a major issue that stems from transmission and can undermine vaccine utility.
Attenuated vaccines can now be designed that largely suppress evolution; recombinant vectors are prone to evolution.
Genetic engineering now enables the design of live viral vaccines that are potentially transmissible. Some designs merely modify a single viral genome to improve on the age-old method of attenuation whereas other designs create chimeras of viral genomes. Transmission has the benefit of increasing herd immunity above that achieved by direct vaccination alone but also increases the opportunity for vaccine evolution, which typically undermines vaccine utility. Different designs have different epidemiological consequences but also experience different evolution. Approaches that integrate vaccine engineering with an understanding of evolution and epidemiology will reap the greatest benefit from vaccine transmission.Vaccine Transmission Is Easier than We Think. Do We Know How toManage It?Many viral vaccines in use, and even more being planned, use live viruses [1–4]. Whenintroduced into a patient, they establish a subdued infection that spreads among cells ofthe host, the infection being sufficient to elicit immunity but not cause disease. Being live, these vaccines are, in principle, capable of transmission to new hosts…” [A2-1]
The researchers state clearly that disease transmission occurs between vaccinated and non-vaccinated people, and even cite solid research that polio is communicated to non-vaccinated people from the vaccinated.
“ Little attention has been given to vaccine transmission, possibly because transmission is rarely measured and largely unknown in humans except for the oral polio vaccine [3,5,6]. Whether transmission is indeed rare for other live vaccines, or has merely gone unnoticed, is not clear –polio vaccine transmission is accompanied by evolution to high virulence, creating problemsthat draw attention to transmission.” [A2-1]
Jannsen / Johnson & Johnson, AstraZeneca and Sinopharm are all manufacturing formulas with attentuated viruses. This means they are capable of being developed as self-transmitting vaccines.
Again from above
“Two types of live viral vaccine are amenable to transmission: attenuated and recombinant vector vaccines.
Attenuated vaccines can now be designed that largely suppress evolution; recombinant vectors are prone to evolution.”
This is from “Non-viral COVID-19 vaccine delivery systems” at NIH:
“This vaccine type can be split into two major types: live attenuated and live inactivated whole virus. Inactivated viruses are more commonly used due to their inability to induce viral reversion. Spearheaded by researchers in China, Wuhan Institute of Biological Products/Sinopharm and Sinovac Biotech have successfully proceeded to Phase III clinical trials using inactivated whole SARS-CoV-2 [7]. In addition, there is a vaccine based on chimpanzee adenovirus called ChAdOx1 developed by the University of Oxford and AstraZeneca as well as Adenovirus 26 vector-based vaccines of Johnson & Johnson that elicit potent immune responses.” [A2-2]
Pfizer/BioNTech’s and Moderna’s formulas are engineered on a mRNA model. Which means it is capable of being developed as a self-transmitting vaccine.
Again from above
“Two types of live viral vaccine are amenable to transmission: attenuated and recombinant vector vaccines.
Recombinant vector vaccines offer the greatest and least appreciated potential for transmission.”
–
This is from “mRNA vaccines — a new era in vaccinology” at NIH:
“mRNA is produced by in vitro reactions with recombinant enzymes, ribonucleotide triphosphates (NTPs) and a DNA template; thus, it is rapid and relatively simple to produce in comparison with traditional protein subunit and live or inactivated virus vaccine production platforms.” [A2-3]
Sources
[A2-1] www.sciencedirect.com/science/article/pii/S0966842X17302123
[A2-2] www.ncbi.nlm.nih.gov/pmc/articles/PMC7744276/
[A2-3] www.ncbi.nlm.nih.gov/pmc/articles/PMC5906799/
|
As we know, a group of people owns almost all major Pharma companies, online companies, financial intuitions, scientific establishments, and they also control the biggest organisations as the World Health Organisation, World Economic Forum, Federal Drug Administration. They also have strong influences on every government! There have been documented news that everything around the Covid19 virus and the PCR tests is inaccurate. Which makes it obvious that this Pandemic has been CREATED FOR A PURPOSE! We also know, some of these people have intentions of depopulating humanity for reasons like fixing environmental problems! If their idea was ‘depopulation through vaccination’, would these SAME people behind this planned pandemic like to get rid of all the vaccinated people and remain with the unvaccinated ones?
OR, if they could find the way, would they like to get rid of everybody else and remain with a certain number of vaccinated, mostly young, healthy, white people from selected countries?
Some of us think the REASON for this mass vaccination is to destroy the economies of the countries, damaging the health of the vaccinated people, their genetical modification and even their death. Yes! But that would RESULT in the survival of unvaccinated people, who are mostly anti system, open minded and not suitable for future slaves! News and fears circulating the internet about the vaccinated people becoming virus-producing/shedding/transmitting machines that will kill themselves and people around them, have truth in them! But this will RESULT in a RANDOM depopulation!
What if there is something EVEN WORSE behind this unending pandemic? Thinking that the people behind this pandemic are malefic, wouldn’t they like to be able to find a way to DIVIDE the people into age/health/occupation groups, and SELECT THE GROUPS that they want to KEEP ALIVE? And also DECIDE THE NUMBER of survivors? The World Health Organisation Vaccination Program is the perfect tool to achieve a SELECTIVE GENOCIDE, rather than a RANDOM one!
We are talking about a situation which IS NOT GOOD EITHER IF YOU VACCINATE or YOU DON’T VACCINATE, EITHER IF YOU DIE or IF YOU LIVE! The only truth we have to concentrate on now is that we didn’t even need to be forced to make the choice of GETTING VACCINATED OR NOT GETTING VACCINATED! This is a FALSE CHOICE in this created pandemic, as an answer to a FALSE PROBLEM! As long as we are caught in this choice (it doesn’t matter if we choose to get vaccinated or not), we are in a NO WAY OUT situation!
NOW, PLEASE, THINK
YOU ARE ONE OF THESE PERSONS WHO IS BEHIND THIS PANDEMIC AND TRY TO ANSWER THIS QUESTION:
HOW WOULD YOU KEEP ALIVE a target number of people, mostly under 65 years old, without chronic diseases or handicaps, from selected countries?
and, at the same time, HOW WOULD YOU ELIMINATE ALL UNVACCINATED PEOPLE, most of the world’s governmental authorities, the elderly, the sick, the handicapped and the homeless?
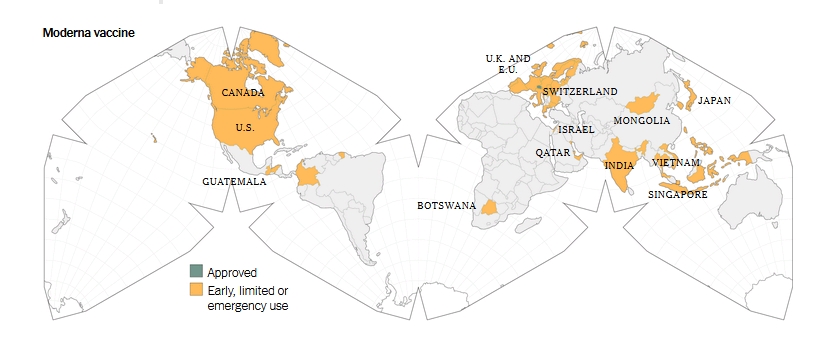
HERE IS HOW IT WOULD BE POSSIBLE:
IF ONE of the anti-Covid19 vaccine – Moderna – is immunising some of the world population ALSO against a FUTURE deadly virus! And if this vaccine is MOSTLY GIVEN to the DESIRED categories of people (young, healthy, white and from selected countries) and NOT GIVEN to the UNDESIRED categories (old, sick, government authorities, homeless)! Every day, old and sick people die more than the young and this fact is used as a reason to separate people, to give priority for vaccination to the undesired categories, so that they DON’T GET the ’special vaccine’!
When that virus will be released in the FUTURE, first, ALL THE UNVACCINATED PEOPLE would be eliminated, along with the UNDESIRED CATEGORIES, and the result would be: ’Reducing of the world population down to approximate desired numbers, resetting all political, juridical, financial and medical systems, eliminating almost all of the world’s authorities, erasing all governments!’ Elite’s long time dream (the One World Government in their new depopulated Earth) would become reality just by USING VACCINATION during this pandemic, to PROTECT A PART OF THE POPULATION by vaccinating it with a special vaccine to survive against a future deadly danger!
|